Steam Reheat on:
[Wikipedia]
[Google]
[Amazon]
 The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain
The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain
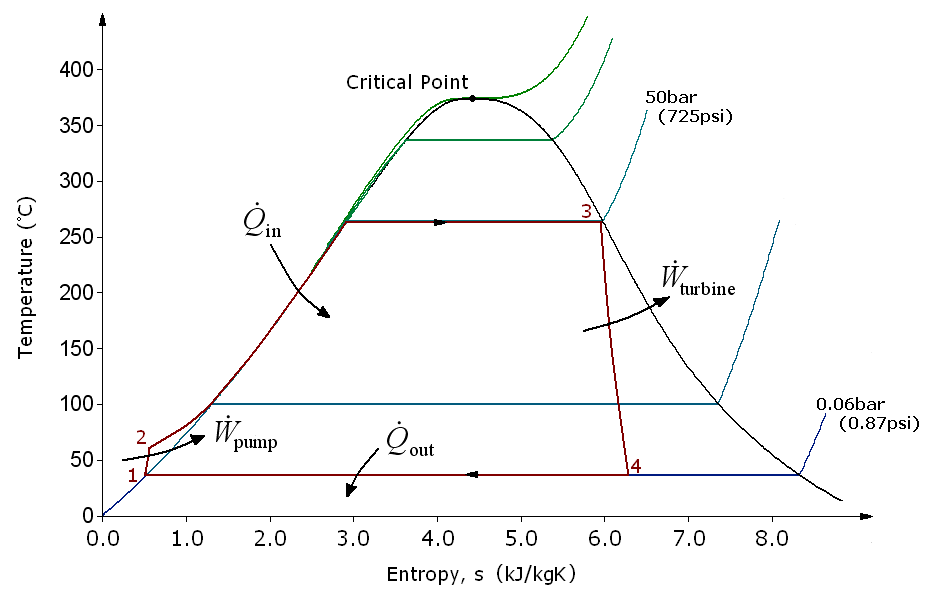 There are four processes in the Rankine cycle. The states are identified by numbers (in brown) in the T–s diagram.
*Process 1–2: The working fluid is pumped from low to high pressure. As the fluid is a liquid at this stage, the pump requires little input energy. Process 1-2 is isentropic compression.
*Process 2–3: The high-pressure liquid enters a boiler, where it is heated at constant pressure by an external heat source to become a dry saturated vapour. The input energy required can be easily calculated graphically, using an enthalpy–entropy chart ( h–s chart, or
There are four processes in the Rankine cycle. The states are identified by numbers (in brown) in the T–s diagram.
*Process 1–2: The working fluid is pumped from low to high pressure. As the fluid is a liquid at this stage, the pump requires little input energy. Process 1-2 is isentropic compression.
*Process 2–3: The high-pressure liquid enters a boiler, where it is heated at constant pressure by an external heat source to become a dry saturated vapour. The input energy required can be easily calculated graphically, using an enthalpy–entropy chart ( h–s chart, or
 In a real power-plant cycle (the name "Rankine" cycle is used only for the ideal cycle), the compression by the pump and the expansion in the
In a real power-plant cycle (the name "Rankine" cycle is used only for the ideal cycle), the compression by the pump and the expansion in the
 The purpose of a reheating cycle is to remove the moisture carried by the steam at the final stages of the expansion process. In this variation, two
The purpose of a reheating cycle is to remove the moisture carried by the steam at the final stages of the expansion process. In this variation, two
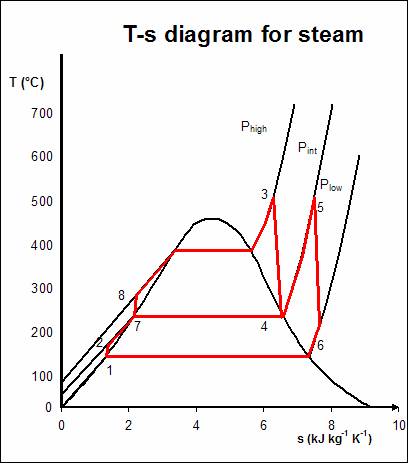 The regenerative Rankine cycle is so named because after emerging from the condenser (possibly as a subcooled liquid) the working fluid is heated by steam tapped from the hot portion of the cycle. On the diagram shown, the fluid at 2 is mixed with the fluid at 4 (both at the same pressure) to end up with the saturated liquid at 7. This is called "direct-contact heating". The Regenerative Rankine cycle (with minor variants) is commonly used in real power stations.
Another variation sends ''bleed steam'' from between turbine stages to
The regenerative Rankine cycle is so named because after emerging from the condenser (possibly as a subcooled liquid) the working fluid is heated by steam tapped from the hot portion of the cycle. On the diagram shown, the fluid at 2 is mixed with the fluid at 4 (both at the same pressure) to end up with the saturated liquid at 7. This is called "direct-contact heating". The Regenerative Rankine cycle (with minor variants) is commonly used in real power stations.
Another variation sends ''bleed steam'' from between turbine stages to
Wikibooks Engineering Thermodynamics
{{Authority control Thermodynamic cycles Scottish inventions
 The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain
The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state ...
s, such as steam turbine
A steam turbine is a machine that extracts thermal energy from pressurized steam and uses it to do mechanical work on a rotating output shaft. Its modern manifestation was invented by Charles Parsons in 1884. Fabrication of a modern steam turbin ...
s or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink
A heat sink (also commonly spelled heatsink) is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant, where it is dissipated away from the device, t ...
. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University.
Heat energy is supplied to the system via a boiler
A boiler is a closed vessel in which fluid (generally water) is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including water heating, central h ...
where the working fluid
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, a ...
(typically water) is converted to a high pressure gaseous state (steam) in order to turn a turbine
A turbine ( or ) (from the Greek , ''tyrbē'', or Latin ''turbo'', meaning vortex) is a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work. The work produced by a turbine can be used for generating e ...
. After passing over the turbine the fluid is allowed to condense back into a liquid state as waste heat energy is rejected before being returned to boiler, completing the cycle. Friction losses throughout the system are often neglected for the purpose of simplifying calculations as such losses are usually much less significant than thermodynamic losses, especially in larger systems.
Description
The Rankine cycle closely describes the process by which steam engines commonly found in thermal power generation plants harness the thermal energy of a fuel or other heat source to generate electricity. Possible heat sources include combustion of fossil fuels such ascoal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when dead ...
, natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon di ...
, and oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturated ...
, use of mined resources for nuclear fission, renewable fuels like biomass and ethanol, or energy capture of natural sources such as concentrated solar power and geothermal energy. Common heat sinks include ambient air above or around a facility and bodies of water such as rivers, ponds, and oceans.
The ability of a Rankine engine to harness energy depends on the relative temperature difference between the heat source and heat sink. The greater the differential, the more mechanical power can be efficiently extracted out of heat energy, as per Carnot's theorem.
The efficiency of the Rankine cycle is limited by the high heat of vaporization of the working fluid. Unless the pressure and temperature reach super critical
''Super Critical'' is the third studio album by English indie pop duo The Ting Tings, released on 24 October 2014 by Finca Records. "Wrong Club" was released as the lead single from the album on 15 August 2014, followed by "Do It Again" on 22 Se ...
levels in the boiler, the temperature range that the cycle can operate over is quite small: Steam turbine entry temperatures are typically around 565 °C and condenser temperatures are around 30 °C. This gives a theoretical maximum Carnot efficiency
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynam ...
for the turbine alone of about 63.8% compared with an actual overall thermal efficiency of less than 50% for typical power stations. This low steam turbine entry temperature (compared to a gas turbine) is why the Rankine (steam) cycle is often used as a bottoming cycle to recover otherwise rejected heat in combined-cycle gas turbine
A combined cycle power plant is an assembly of heat engines that work in tandem from the same source of heat, converting it into mechanical energy. On land, when used to make electricity the most common type is called a combined cycle gas tur ...
power stations.
Rankine engines generally operate in a closed loop where the working fluid is reused. The water vapor
In physics, a vapor (American English) or vapour (British English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Her ...
with condensed droplets often seen billowing from power stations is created by the cooling systems (not directly from the closed-loop Rankine power cycle). This 'exhaust' heat is represented by the "Qout" flowing out of the lower side of the cycle shown in the T–s diagram below. Cooling tower
A cooling tower is a device that rejects waste heat to the atmosphere through the cooling of a coolant stream, usually a water stream to a lower temperature. Cooling towers may either use the evaporation of water to remove process heat and c ...
s operate as large heat exchangers by absorbing the latent heat of vaporization of the working fluid and simultaneously evaporating cooling water to the atmosphere.
While many substances can be used as the working fluid, water is usually chosen for its simple chemistry, relative abundance, low cost, and thermodynamic properties
In thermodynamics, a physical property is any property that is measurable, and whose value describes a state of a physical system. Thermodynamic properties are defined as characteristic features of a system, capable of specifying the system's stat ...
. By condensing the working steam vapor to a liquid the pressure at the turbine outlet is lowered and the energy required by the feed pump consumes only 1% to 3% of the turbine output power and these factors contribute to a higher efficiency for the cycle. The benefit of this is offset by the low temperatures of steam admitted to the turbine(s). Gas turbines, for instance, have turbine entry temperatures approaching 1500 °C. However, the thermal efficiency of actual large steam power stations and large modern gas turbine stations are similar.
The four processes in the Rankine cycle
 There are four processes in the Rankine cycle. The states are identified by numbers (in brown) in the T–s diagram.
*Process 1–2: The working fluid is pumped from low to high pressure. As the fluid is a liquid at this stage, the pump requires little input energy. Process 1-2 is isentropic compression.
*Process 2–3: The high-pressure liquid enters a boiler, where it is heated at constant pressure by an external heat source to become a dry saturated vapour. The input energy required can be easily calculated graphically, using an enthalpy–entropy chart ( h–s chart, or
There are four processes in the Rankine cycle. The states are identified by numbers (in brown) in the T–s diagram.
*Process 1–2: The working fluid is pumped from low to high pressure. As the fluid is a liquid at this stage, the pump requires little input energy. Process 1-2 is isentropic compression.
*Process 2–3: The high-pressure liquid enters a boiler, where it is heated at constant pressure by an external heat source to become a dry saturated vapour. The input energy required can be easily calculated graphically, using an enthalpy–entropy chart ( h–s chart, or Mollier diagram Mollier may refer to:
* Richard Mollier, German professor of Applied Physics and Mechanics ;
* Louis-Marie Mollier, French-American pioneer priest of north-central Kansas ;
* Jean-Yves Mollier
Jean-Yves Mollier (born 5 November 1947) is a Fren ...
), or numerically, using steam tables or software. Process 2-3 is constant pressure heat addition in boiler.
*Process 3–4: The dry saturated vapour expands through a turbine
A turbine ( or ) (from the Greek , ''tyrbē'', or Latin ''turbo'', meaning vortex) is a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work. The work produced by a turbine can be used for generating e ...
, generating power. This decreases the temperature and pressure of the vapour, and some condensation may occur. The output in this process can be easily calculated using the chart or tables noted above. Process 3-4 is isentropic expansion.
*Process 4–1: The wet vapour then enters a condenser, where it is condensed at a constant pressure to become a saturated liquid
Saturation, saturated, unsaturation or unsaturated may refer to:
Chemistry
* Saturation, a property of organic compounds referring to carbon-carbon bonds
**Saturated and unsaturated compounds
** Degree of unsaturation
**Saturated fat or fatty aci ...
. Process 4-1 is constant pressure heat rejection in condenser.
In an ideal Rankine cycle the pump and turbine would be isentropic, i.e., the pump and turbine would generate no entropy and hence maximize the net work output. Processes 1–2 and 3–4 would be represented by vertical lines on the T–s diagram and more closely resemble that of the Carnot cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodyna ...
. The Rankine cycle shown here prevents the state of the working fluid from ending up in the superheated vapor region after the expansion in the turbine,
which reduces the energy removed by the condensers.
The actual vapor power cycle differs from the ideal Rankine cycle because of irreversibilities in the inherent components caused by fluid friction and heat loss to the surroundings; fluid friction causes pressure drops in the boiler, the condenser, and the piping between the components, and as a result the steam leaves the boiler at a lower pressure; heat loss reduces the net work output, thus heat addition to the steam in the boiler is required to maintain the same level of net work output.
Variables
Equations
In general, the efficiency of a simple rankine cycle can be written as : Each of the next four equations is derived from the energy andmass balance
In physics, a mass balance, also called a material balance, is an application of conservation of mass to the analysis of physical systems. By accounting for material entering and leaving a system, mass flows can be identified which might have b ...
for a control volume. defines the thermodynamic efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
For a h ...
of the cycle as the ratio of net power output to heat input. As the work required by the pump is often around 1% of the turbine work output, it can be simplified.
:
:
:
:
When dealing with the efficiencies of the turbines and pumps, an adjustment to the work terms must be made:
:
:
Real Rankine cycle (non-ideal)
 In a real power-plant cycle (the name "Rankine" cycle is used only for the ideal cycle), the compression by the pump and the expansion in the
In a real power-plant cycle (the name "Rankine" cycle is used only for the ideal cycle), the compression by the pump and the expansion in the turbine
A turbine ( or ) (from the Greek , ''tyrbē'', or Latin ''turbo'', meaning vortex) is a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work. The work produced by a turbine can be used for generating e ...
are not isentropic. In other words, these processes are non-reversible, and entropy is increased during the two processes. This somewhat increases the power
Power most often refers to:
* Power (physics), meaning "rate of doing work"
** Engine power, the power put out by an engine
** Electric power
* Power (social and political), the ability to influence people or events
** Abusive power
Power may ...
required by the pump and decreases the power generated by the turbine.
In particular, the efficiency of the steam turbine will be limited by water-droplet formation. As the water condenses, water droplets hit the turbine blades at high speed, causing pitting and erosion, gradually decreasing the life of turbine blades and efficiency of the turbine. The easiest way to overcome this problem is by superheating the steam. On the T–s diagram above, state 3 is at a border of the two-phase region of steam and water, so after expansion the steam will be very wet. By superheating, state 3 will move to the right (and up) in the diagram and hence produce a drier steam after expansion.
Variations of the basic Rankine cycle
The overallthermodynamic efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
For a h ...
can be increased by raising the average heat input temperature
:
of that cycle. Increasing the temperature of the steam into the superheat region is a simple way of doing this. There are also variations of the basic Rankine cycle designed to raise the thermal efficiency of the cycle in this way; two of these are described below.
Rankine cycle with reheat
 The purpose of a reheating cycle is to remove the moisture carried by the steam at the final stages of the expansion process. In this variation, two
The purpose of a reheating cycle is to remove the moisture carried by the steam at the final stages of the expansion process. In this variation, two turbine
A turbine ( or ) (from the Greek , ''tyrbē'', or Latin ''turbo'', meaning vortex) is a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work. The work produced by a turbine can be used for generating e ...
s work in series. The first accepts vapor
In physics, a vapor (American English) or vapour (British English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Her ...
from the boiler
A boiler is a closed vessel in which fluid (generally water) is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including water heating, central h ...
at high pressure. After the vapor has passed through the first turbine, it re-enters the boiler and is reheated before passing through a second, lower-pressure, turbine. The reheat temperatures are very close or equal to the inlet temperatures, whereas the optimal reheat pressure needed is only one fourth of the original boiler pressure. Among other advantages, this prevents the vapor from condensing during its expansion and thereby reducing the damage in the turbine blades, and improves the efficiency of the cycle, because more of the heat flow into the cycle occurs at higher temperature. The reheat cycle was first introduced in the 1920s, but was not operational for long due to technical difficulties. In the 1940s, it was reintroduced with the increasing manufacture of high-pressure boiler
A boiler is a closed vessel in which fluid (generally water) is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including water heating, central h ...
s, and eventually double reheating was introduced in the 1950s. The idea behind double reheating is to increase the average temperature. It was observed that more than two stages of reheating are generally unnecessary, since the next stage increases the cycle efficiency only half as much as the preceding stage. Today, double reheating is commonly used in power plants that operate under supercritical pressure.
Regenerative Rankine cycle
 The regenerative Rankine cycle is so named because after emerging from the condenser (possibly as a subcooled liquid) the working fluid is heated by steam tapped from the hot portion of the cycle. On the diagram shown, the fluid at 2 is mixed with the fluid at 4 (both at the same pressure) to end up with the saturated liquid at 7. This is called "direct-contact heating". The Regenerative Rankine cycle (with minor variants) is commonly used in real power stations.
Another variation sends ''bleed steam'' from between turbine stages to
The regenerative Rankine cycle is so named because after emerging from the condenser (possibly as a subcooled liquid) the working fluid is heated by steam tapped from the hot portion of the cycle. On the diagram shown, the fluid at 2 is mixed with the fluid at 4 (both at the same pressure) to end up with the saturated liquid at 7. This is called "direct-contact heating". The Regenerative Rankine cycle (with minor variants) is commonly used in real power stations.
Another variation sends ''bleed steam'' from between turbine stages to feedwater heater
A feedwater heater is a power plant component used to pre-heat water delivered to a steam generating boiler. Preheating the feedwater reduces the irreversibilities involved in steam generation and therefore improves the thermodynamic efficiency of ...
s to preheat the water on its way from the condenser to the boiler. These heaters do not mix the input steam and condensate, function as an ordinary tubular heat exchanger, and are named "closed feedwater heaters".
Regeneration increases the cycle heat input temperature by eliminating the addition of heat from the boiler/fuel source at the relatively low feedwater temperatures that would exist without regenerative feedwater heating. This improves the efficiency of the cycle, as more of the heat flow into the cycle occurs at higher temperature.
Organic Rankine cycle
The organic Rankine cycle (ORC) uses an organic fluid such asn-pentane
Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the '' ...
or toluene in place of water and steam. This allows use of lower-temperature heat sources, such as solar ponds, which typically operate at around 70 –90 °C.Nielsen et al., 2005, Proc. Int. Solar Energy Soc. The efficiency
Efficiency is the often measurable ability to avoid wasting materials, energy, efforts, money, and time in doing something or in producing a desired result. In a more general sense, it is the ability to do things well, successfully, and without ...
of the cycle is much lower as a result of the lower temperature range, but this can be worthwhile because of the lower cost involved in gathering heat at this lower temperature. Alternatively, fluids can be used that have boiling points above water, and this may have thermodynamic benefits (See, for example, mercury vapour turbine A mercury vapour turbine is a form of heat engine that uses mercury as the working fluid of its thermal cycle. A mercury vapour turbine has been used in conjunction with a steam turbine for generating electricity. This example of combined cycle ...
). The properties of the actual working fluid have great influence on the quality of steam (vapour) after the expansion step, influencing the design of the whole cycle.
The Rankine cycle does not restrict the working fluid in its definition, so the name "organic cycle" is simply a marketing concept and the cycle should not be regarded as a separate thermodynamic cycle.
Supercritical Rankine cycle
The Rankine cycle applied using a supercritical fluid combines the concepts of heat regeneration and supercritical Rankine cycle into a unified process called the regenerative supercritical cycle (RGSC). It is optimised for temperature sources 125–450 °C.See also
*Brayton cycle
The Brayton cycle is a thermodynamic cycle that describes the operation of certain heat engines that have air or some other gas as their working fluid. The original Brayton engines used a piston compressor and piston expander, but modern gas tur ...
* Power loss in cogeneration mode with steam extraction
References
* ^Van Wyllen 'Fundamentals of thermodynamics' () * ^Wong 'Thermodynamics for Engineers',2nd Ed.,2012, CRC Press, Taylor & Francis, Boca Raton, London, New York. () *Moran & Shapiro 'Fundamentals of Engineering Thermodynamics' ()Wikibooks Engineering Thermodynamics
{{Authority control Thermodynamic cycles Scottish inventions