spin forbidden reactions on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, the selection rule (also known as the transition rule) formally restricts certain reactions, known as spin-forbidden reactions, from occurring due to a required change between two differing
 Singlet and triplet states can occur within organometallic complexes as well, such as Tp''i''-Pr,MeCo(CO)2 and Tp''i''-Pr,MeCo(CO), respectively.
Singlet and triplet states can occur within organometallic complexes as well, such as Tp''i''-Pr,MeCo(CO)2 and Tp''i''-Pr,MeCo(CO), respectively.

 Once a minimum energy crossing point is reached and parameter 1 above is satisfied, the system needs to hop from one diabatic surface to the other, as stated above by parameter 2. At a given energy (''E''), the rate coefficient 'k(E)''of a spin-forbidden reaction can be calculated using the density of rovibrational states of the reactant 'ρ(E)''and the effective integrated density of states in the crossing seam between the two surfaces 'Ner(E)''
:
where
:
The probability of hopping (''psh'') is calculated from Landau-Zener theory giving
:
where
:
in which the spin-orbit coupling derived off the diagonal Hamiltonian matrix element between two electronic states (''H12''), the relative slope of the two surfaces at the crossing seam 'F(Δ)'' the reduced mass of the system through its movement along the hopping coordinate (''μ''), and the kinetic energy of the system passing through the crossing point (''E'') are used.
It is useful to note that when ''Eh'' < ''Ec'' (when below the minimum energy crossing point) the probability of hopping between spin states is null.
Once a minimum energy crossing point is reached and parameter 1 above is satisfied, the system needs to hop from one diabatic surface to the other, as stated above by parameter 2. At a given energy (''E''), the rate coefficient 'k(E)''of a spin-forbidden reaction can be calculated using the density of rovibrational states of the reactant 'ρ(E)''and the effective integrated density of states in the crossing seam between the two surfaces 'Ner(E)''
:
where
:
The probability of hopping (''psh'') is calculated from Landau-Zener theory giving
:
where
:
in which the spin-orbit coupling derived off the diagonal Hamiltonian matrix element between two electronic states (''H12''), the relative slope of the two surfaces at the crossing seam 'F(Δ)'' the reduced mass of the system through its movement along the hopping coordinate (''μ''), and the kinetic energy of the system passing through the crossing point (''E'') are used.
It is useful to note that when ''Eh'' < ''Ec'' (when below the minimum energy crossing point) the probability of hopping between spin states is null.
 :
:Fe(CO)2->[][CO]Fe(CO)3->[][CO]Fe(CO)4->[][CO]Fe(CO)5
where:
:,
:, and
:.
The rate constants above are notably temperature independent, tested at 55 °C, 21 °C, and 10 °C, indicating, according to the authors, that the observed 500 fold reduction in rate occurs due to the spin-forbidden nature of the latter equation and not due to the kinetics of adding an additional ligand.

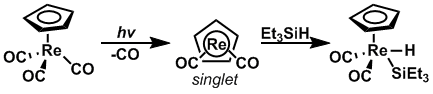
 Metal-oxo species, due to their small spatial extent of metal-centered ''d'' orbitals leading to weak bonding, often have similar energies for both the low spin (
Metal-oxo species, due to their small spatial extent of metal-centered ''d'' orbitals leading to weak bonding, often have similar energies for both the low spin (M=O ) and high spin configuration (*M-O* ). This similarity in energy between the low- and high spin configurations of oxo-species lends itself to the study of spin-forbidden reactions, such as Mn(salen)-catalyzed epoxidation. The Mn(salen)-oxo species can exist in either a triplet or quintet state. While the product of the quintet lies at a lower energy, both the triplet and quintet products can be observed.{{cite journal, last1=Linde, first1=C., last2=Åkermark, first2=B., last3=Norrby, first3=P.-O., last4=Svensson, first4=M., date=1999, title=Timing Is Critical: Effect of Spin Changes on the Diastereoslectivity in Mn(salen)-Catalyzed Epoxidation, journal=Journal of the American Chemical Society, volume=121, issue=21, pages=5083–4, doi=10.1021/ja9809915

quantum state
In quantum physics, a quantum state is a mathematical entity that provides a probability distribution for the outcomes of each possible measurement on a system. Knowledge of the quantum state together with the rules for the system's evolution i ...
s. When a reactant
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
exists in one spin state and the product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
exists in a different spin state, the corresponding reaction will have an increased activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
when compared to a similar reaction in which the spin states of the reactant and product are isomorphic. As a result of this increased activation energy, a decreased rate of reaction
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per unit ...
is observed.
Case of some cobalt carbonyls
 Singlet and triplet states can occur within organometallic complexes as well, such as Tp''i''-Pr,MeCo(CO)2 and Tp''i''-Pr,MeCo(CO), respectively.
Singlet and triplet states can occur within organometallic complexes as well, such as Tp''i''-Pr,MeCo(CO)2 and Tp''i''-Pr,MeCo(CO), respectively.

Changing spin states
When a reaction converts a metal from a singlet to triplet state (or ''vice versa''): # The energy of the two spin states must be nearly equal, as dictated by temperature, # A mechanism is required to change spin states. Strong spin-orbital coupling can satisfy the 2nd condition. Parameter 1, however, can lead to very slow reactions due to large disparities between the metal complex'spotential energy surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinat ...
s, which only cross at high energy leading to a substantial activation barrier.
Spin-forbidden reactions formally fall into the category of ''electronically non-adiabatic reactions''. In general, potential energy surfaces fall into either the adiabatic and diabatic classification. Potential Energy Surfaces that are adiabatic rely on the use of the full electronic Hamiltonian, which includes the spin-orbit term. Those that are diabatic are likewise derived by solving the eigenvalues of the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
, but in this case one or more terms are omitted.
Non-adiabatic transition
 Once a minimum energy crossing point is reached and parameter 1 above is satisfied, the system needs to hop from one diabatic surface to the other, as stated above by parameter 2. At a given energy (''E''), the rate coefficient 'k(E)''of a spin-forbidden reaction can be calculated using the density of rovibrational states of the reactant 'ρ(E)''and the effective integrated density of states in the crossing seam between the two surfaces 'Ner(E)''
:
where
:
The probability of hopping (''psh'') is calculated from Landau-Zener theory giving
:
where
:
in which the spin-orbit coupling derived off the diagonal Hamiltonian matrix element between two electronic states (''H12''), the relative slope of the two surfaces at the crossing seam 'F(Δ)'' the reduced mass of the system through its movement along the hopping coordinate (''μ''), and the kinetic energy of the system passing through the crossing point (''E'') are used.
It is useful to note that when ''Eh'' < ''Ec'' (when below the minimum energy crossing point) the probability of hopping between spin states is null.
Once a minimum energy crossing point is reached and parameter 1 above is satisfied, the system needs to hop from one diabatic surface to the other, as stated above by parameter 2. At a given energy (''E''), the rate coefficient 'k(E)''of a spin-forbidden reaction can be calculated using the density of rovibrational states of the reactant 'ρ(E)''and the effective integrated density of states in the crossing seam between the two surfaces 'Ner(E)''
:
where
:
The probability of hopping (''psh'') is calculated from Landau-Zener theory giving
:
where
:
in which the spin-orbit coupling derived off the diagonal Hamiltonian matrix element between two electronic states (''H12''), the relative slope of the two surfaces at the crossing seam 'F(Δ)'' the reduced mass of the system through its movement along the hopping coordinate (''μ''), and the kinetic energy of the system passing through the crossing point (''E'') are used.
It is useful to note that when ''Eh'' < ''Ec'' (when below the minimum energy crossing point) the probability of hopping between spin states is null.
An example of a spin-forbidden reaction
One example showing the slowing effect of spin-forbidden reaction takes place when Fe(CO)x is placed under CO pressure. Transitions from ''x ''= 2,3,4 to ''x ''= 3,4,5 demonstrate a slowing rate when the reactant is in a triplet ground state but the product is in a singlet ground state. In the case of Fe(CO)x, when ''x'' = 2, 3, 4 the iron exists as a triplet in ground state; when ''x ''= 5, the iron exists as a singlet in ground state. The kinetics of said system can be represented by: :
:Application to catalysis
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
association and dissociation from metal centers involve a fundamental change in the coordination sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ...
of that metal. This change in certain reactions also requires a change in spin state to occur, which can retard the rate of ligand association. Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxid ...
and reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
can be thought of in a similar manner to ligand association and dissociation, respectively. Mathematically, the rate ligand dissociation can increase when the reaction proceeds from one spin state to another, although it must be noted that this effect is often small in comparison with other factors such as sterics around the metal center.
C-H activation
Insertion into C-H bonds, known as C-H activation, is an integral first step in C-H functionalization. For some metal complexes with identical ligands, C-H activation is rapid when one metal is used and slow when other metals are used, often first row transition metals, due to the spin allowed nature of the former case and the spin-forbidden nature of the latter case. The difference in rates of C-H activation of methane for CoCp(CO), RhCp(CO), and IrCp(CO) readily demonstrate this property. CoCp(CO), the starting material in a C-H activation, exists in a triplet spin state while RhCp(CO) exists in a singlet state, with the triplet state only 5.9 kcal/mol away. IrCp(CO) is unique among these complexes in that its starting state is essentially degenerate between the triplet and singlet states. The given product of C-H insertion, CpMH(CO)(CH3), where M = Co, Rh, Ir, is in a singlet state meaning that the C-H activation with CoCp(CO) must reach the minimum energy crossing point for the reactant and product's potential energy surfaces, thus requiring relatively high energies to proceed.
Oxidative addition into silicon-hydrogen bonds
Through the use of photolysis, the rate of oxidative addition intosilicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
-hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
bonds has been shown to increase when the starting material is excited to the correct spin-state. CpRe(CO)2 and CpMn(CO)2 were subjected to photolysis to change their spin states from triplet to singlet and ''vice versa,'' respectively, such that an oxidative addition to Et3Si-H could occur at a greatly accelerated rate.
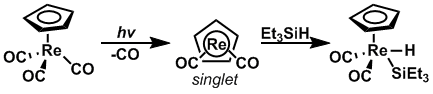
Oxidation chemistry
 Metal-oxo species, due to their small spatial extent of metal-centered ''d'' orbitals leading to weak bonding, often have similar energies for both the low spin (
Metal-oxo species, due to their small spatial extent of metal-centered ''d'' orbitals leading to weak bonding, often have similar energies for both the low spin (
References