radioactive decay on:
[Wikipedia]
[Google]
[Amazon]
 Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable
 Radioactivity was discovered in 1896 by scientists Henri Becquerel and
Radioactivity was discovered in 1896 by scientists Henri Becquerel and
 The dangers of
The dangers of
 However, the biological effects of radiation due to radioactive substances were less easy to gauge. This gave the opportunity for many physicians and corporations to market radioactive substances as
However, the biological effects of radiation due to radioactive substances were less easy to gauge. This gave the opportunity for many physicians and corporations to market radioactive substances as
 The
The
 Early researchers found that an electric or
Early researchers found that an electric or  In analysing the nature of the decay products, it was obvious from the direction of the electromagnetic forces applied to the radiations by external magnetic and electric fields that
In analysing the nature of the decay products, it was obvious from the direction of the electromagnetic forces applied to the radiations by external magnetic and electric fields that

 A number of experiments have found that decay rates of other modes of artificial and naturally occurring radioisotopes are, to a high degree of precision, unaffected by external conditions such as temperature, pressure, the chemical environment, and electric, magnetic, or gravitational fields. Comparison of laboratory experiments over the last century, studies of the Oklo natural nuclear reactor (which exemplified the effects of thermal neutrons on nuclear decay), and astrophysical observations of the luminosity decays of distant supernovae (which occurred far away so the light has taken a great deal of time to reach us), for example, strongly indicate that unperturbed decay rates have been constant (at least to within the limitations of small experimental errors) as a function of time as well.
Recent results suggest the possibility that decay rates might have a weak dependence on environmental factors. It has been suggested that measurements of decay rates of
A number of experiments have found that decay rates of other modes of artificial and naturally occurring radioisotopes are, to a high degree of precision, unaffected by external conditions such as temperature, pressure, the chemical environment, and electric, magnetic, or gravitational fields. Comparison of laboratory experiments over the last century, studies of the Oklo natural nuclear reactor (which exemplified the effects of thermal neutrons on nuclear decay), and astrophysical observations of the luminosity decays of distant supernovae (which occurred far away so the light has taken a great deal of time to reach us), for example, strongly indicate that unperturbed decay rates have been constant (at least to within the limitations of small experimental errors) as a function of time as well.
Recent results suggest the possibility that decay rates might have a weak dependence on environmental factors. It has been suggested that measurements of decay rates of
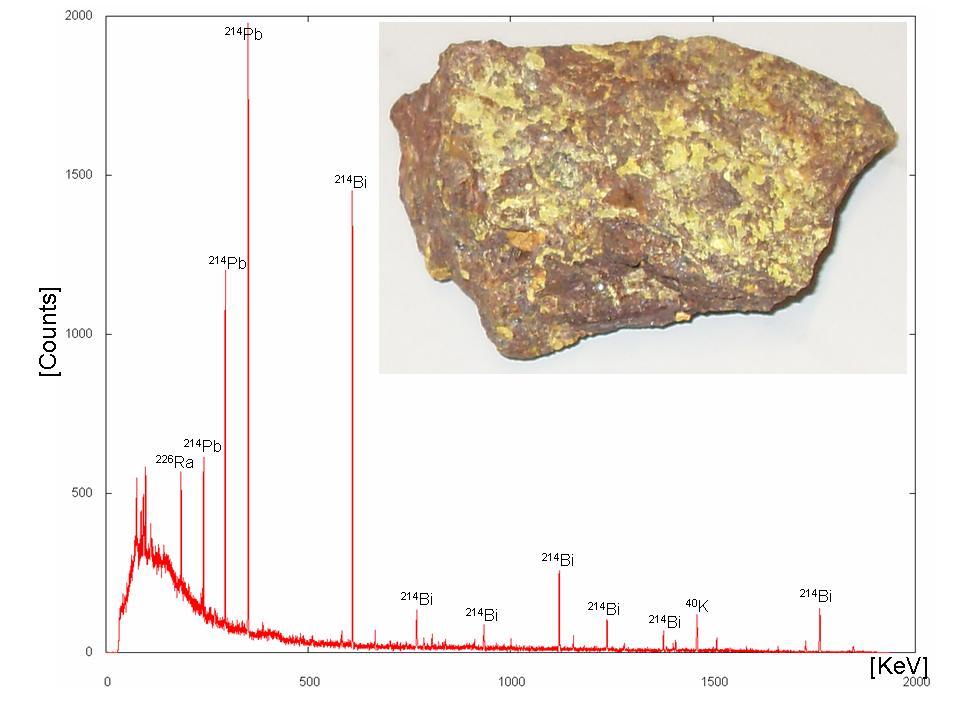 An example is the natural decay chain of 238U:
* Uranium-238 decays, through alpha-emission, with a
An example is the natural decay chain of 238U:
* Uranium-238 decays, through alpha-emission, with a
file:Radioactive.svg, The trefoil symbol used to warn of presence of radioactive material or ionising radiation
File:Logo iso radiation.svg, 2007 ISO radioactivity IAEA news release Feb 2007
/ref> File:Dangclass7.svg, The dangerous goods transport classification sign for radioactive materials
"Radioactivity"
Encyclopædia Britannica. 2006.
The Lund/LBNL Nuclear Data Search
– Contains tabulated information on radioactive decay types and energies.
Nomenclature of nuclear chemistry
The Live Chart of Nuclides – IAEA
Interactive Chart of Nuclides
Health Physics Society Public Education Website
*
Annotated bibliography for radioactivity from the Alsos Digital Library for Nuclear Issues
by Wolfgang Bauer
by David M. Harrison * "Henri Becquerel: The Discovery of Radioactivity", Becquerel's 1896 articles online and analyzed on
BibNum
'
Bibnum
'
atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron ...
loses energy by radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
* ''electromagnetic radiation'', such as radio waves, microwaves, infrared, visi ...
. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particle
In the physical sciences, a particle (or corpuscule in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from ...
s. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism
In physics, electromagnetism is an interaction that occurs between particles with electric charge. It is the second-strongest of the four fundamental interactions, after the strong force, and it is the dominant force in the interactions o ...
and nuclear force. A fourth type of common decay is electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumor ...
commonly used in medical settings.
Radioactive decay is a stochastic
Stochastic (, ) refers to the property of being well described by a random probability distribution. Although stochasticity and randomness are distinct in that the former refers to a modeling approach and the latter refers to phenomena themselv ...
(i.e. random) process at the level of single atoms. According to quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate ...
or as half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
. The half-lives of radioactive atoms have a huge range; from nearly instantaneous to far longer than the age of the universe.
The decaying nucleus is called the ''parent radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
'' (or ''parent radioisotope''Radionuclide is the more correct term, but radioisotope is also used. The difference between isotope and nuclide is explained at .), and the process produces at least one '' daughter nuclide''. Except for gamma decay or internal conversion from a nuclear excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to ...
, the decay is a nuclear transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
A transmutatio ...
resulting in a daughter containing a different number of proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s or neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s (or both). When the number of protons changes, an atom of a different chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
is created.
* Alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
occurs when the nucleus ejects an alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
(helium nucleus).
* Beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
occurs in two ways;
* In gamma decay a radioactive nucleus first decays by the emission of an alpha or beta particle. The daughter nucleus that results is usually left in an excited state and it can decay to a lower energy state by emitting a gamma ray photon.
* In neutron emission, extremely neutron-rich nuclei, formed due to other types of decay or after many successive neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
s, occasionally lose energy by way of neutron emission, resulting in a change from one isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
to another of the same element.
* In electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
, the nucleus may capture an orbiting electron, causing a proton to convert into a neutron. A neutrino and a gamma ray are subsequently emitted.
* In cluster decay
Cluster decay, also named heavy particle radioactivity or heavy ion radioactivity, is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typic ...
and nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
, a nucleus heavier than an alpha particle is emitted.
By contrast there are radioactive decay processes that do not result in a nuclear transmutation. The energy of an excited nucleus may be emitted as a gamma ray in a process called gamma decay, or that energy may be lost when the nucleus interacts with an orbital electron causing its ejection from the atom, in a process called internal conversion. Another type of radioactive decay results in products that vary, appearing as two or more "fragments" of the original nucleus with a range of possible masses. This decay, called spontaneous fission, happens when a large unstable nucleus spontaneously splits into two (or occasionally three) smaller daughter nuclei, and generally leads to the emission of gamma rays, neutrons, or other particles from those products.
In contrast, decay products from a nucleus ''with spin'' may be distributed ''non-isotropically'' with respect to that spin direction. Either because of an external influence such as an electromagnetic field
An electromagnetic field (also EM field or EMF) is a classical (i.e. non-quantum) field produced by (stationary or moving) electric charges. It is the field described by classical electrodynamics (a classical field theory) and is the classical ...
, or because the nucleus was produced in a dynamic process that constrained the direction of its spin, the anisotropy may be detectable. Such a parent process could be a previous decay, or a nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformatio ...
.See Wu experiment among other counterexamples when the decaying atom is influenced by external factors.
For a summary table showing the number of stable and radioactive nuclides, see radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
. There are 28 naturally occurring chemical elements on Earth that are radioactive, consisting of 34 radionuclides (6 elements have 2 different radionuclides) that date before the time of formation of the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
. These 34 are known as primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
s. Well-known examples are uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
, but also included are naturally occurring long-lived radioisotopes, such as potassium-40.
Another 50 or so shorter-lived radionuclides found on Earth such as radium-226 and radon-222, are the products of decay chains that began with the primordial nuclides, or are the product of ongoing cosmogenic processes, such as the production of carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and co ...
from nitrogen-14
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15. Fourteen radioisotopes are also known, with atomic masses ranging from 10 to 2 ...
in the atmosphere by cosmic rays
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our ...
. Radionuclides may also be produced artificially in particle accelerators
A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams.
Large accelerators are used for fundamental research in particle ...
or nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from ...
, resulting in 650 of these with half-lives of over an hour, and several thousand more with even shorter half-lives. (See List of nuclides for a list of these sorted by half-life.)
History of discovery
 Radioactivity was discovered in 1896 by scientists Henri Becquerel and
Radioactivity was discovered in 1896 by scientists Henri Becquerel and Marie Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the fir ...
, while working with phosphorescent materials. These materials glow in the dark after exposure to light, and he suspected that the glow produced in cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), ...
s by X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
s might be associated with phosphorescence. Becquerel wrapped a photographic plate in black paper and placed various phosphorescent salts on it. All results were negative until he used uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
salts. The uranium salts caused a blackening of the plate in spite of the plate being wrapped in black paper. These radiations were given the name "Becquerel Rays".
It soon became clear that the blackening of the plate had nothing to do with phosphorescence, as the blackening was also produced by non-phosphorescent salts of uranium and by metallic uranium. It became clear from these experiments that there was a form of invisible radiation that could pass through paper and was causing the plate to react as if exposed to light.
At first, it seemed as though the new radiation was similar to the then recently discovered X-rays. Further research by Becquerel, Ernest Rutherford, Paul Villard, Pierre Curie
Pierre Curie ( , ; 15 May 1859 – 19 April 1906) was a French physicist, a pioneer in crystallography, magnetism, piezoelectricity, and radioactivity. In 1903, he received the Nobel Prize in Physics with his wife, Marie Curie, and Henri Becq ...
, Marie Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the fir ...
, and others showed that this form of radioactivity was significantly more complicated. Rutherford was the first to realize that all such elements decay in accordance with the same mathematical exponential formula. Rutherford and his student Frederick Soddy were the first to realize that many decay processes resulted in the transmutation of one element to another. Subsequently, the radioactive displacement law of Fajans and Soddy
The law of radioactive displacements, also known as Fajans's and Soddy's law, in radiochemistry and nuclear physics, is a rule governing the transmutation of elements during radioactive decay. It is named after Frederick Soddy and Kazimierz Fajans ...
was formulated to describe the products of alpha
Alpha (uppercase , lowercase ; grc, ἄλφα, ''álpha'', or ell, άλφα, álfa) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter aleph , whi ...
and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
.
The early researchers also discovered that many other chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
s, besides uranium, have radioactive isotopes. A systematic search for the total radioactivity in uranium ores also guided Pierre and Marie Curie to isolate two new elements: polonium and radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rat ...
. Except for the radioactivity of radium, the chemical similarity of radium to barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
made these two elements difficult to distinguish.
Marie and Pierre Curie's study of radioactivity is an important factor in science and medicine. After their research on Becquerel's rays led them to the discovery of both radium and polonium, they coined the term "radioactivity" to define the emission of ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
by some heavy elements. (Later the term was generalized to all elements.) Their research on the penetrating rays in uranium and the discovery of radium launched an era of using radium for the treatment of cancer. Their exploration of radium could be seen as the first peaceful use of nuclear energy and the start of modern nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
.
Early health dangers
 The dangers of
The dangers of ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
due to radioactivity and X-rays were not immediately recognized.
X-rays
The discovery of X‑rays byWilhelm Röntgen
Wilhelm Conrad Röntgen (; ; 27 March 184510 February 1923) was a German mechanical engineer and physicist, who, on 8 November 1895, produced and detected electromagnetic radiation in a wavelength range known as X-rays or Röntgen rays, an achie ...
in 1895 led to widespread experimentation by scientists, physicians, and inventors. Many people began recounting stories of burns, hair loss and worse in technical journals as early as 1896. In February of that year, Professor Daniel and Dr. Dudley of Vanderbilt University
Vanderbilt University (informally Vandy or VU) is a private research university in Nashville, Tennessee. Founded in 1873, it was named in honor of shipping and rail magnate Cornelius Vanderbilt, who provided the school its initial $1-million ...
performed an experiment involving X-raying Dudley's head that resulted in his hair loss. A report by Dr. H.D. Hawks, of his suffering severe hand and chest burns in an X-ray demonstration, was the first of many other reports in ''Electrical Review''.
Other experimenters, including Elihu Thomson and Nikola Tesla
Nikola Tesla ( ; ,"Tesla"
''Random House Webster's Unabridged Dictionary''. ; 1856 – 7 January 1943 ...
, also reported burns. Thomson deliberately exposed a finger to an X-ray tube over a period of time and suffered pain, swelling, and blistering. Other effects, including ultraviolet rays and ozone, were sometimes blamed for the damage, and many physicians still claimed that there were no effects from X-ray exposure at all.
Despite this, there were some early systematic hazard investigations, and as early as 1902 ''Random House Webster's Unabridged Dictionary''. ; 1856 – 7 January 1943 ...
William Herbert Rollins
William Herbert Rollins (June 19, 1852 - 1929) was an American scientist, inventor, and dentist. He was a pioneer in radiation protection. Many of his inventions and investigations in medical radiography and photography have been ranked in import ...
wrote almost despairingly that his warnings about the dangers involved in the careless use of X-rays were not being heeded, either by industry or by his colleagues. By this time, Rollins had proved that X-rays could kill experimental animals, could cause a pregnant guinea pig to abort, and that they could kill a foetus. He also stressed that "animals vary in susceptibility to the external action of X-light" and warned that these differences be considered when patients were treated by means of X-rays.
Radioactive substances
patent medicine
A patent medicine, sometimes called a proprietary medicine, is an over-the-counter (nonprescription) medicine or medicinal preparation that is typically protected and advertised by a trademark and trade name (and sometimes a patent) and claimed ...
s. Examples were radium enema treatments, and radium-containing waters to be drunk as tonics. Marie Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the fir ...
protested against this sort of treatment, warning that "radium is dangerous in untrained hands." Curie later died from aplastic anaemia, likely caused by exposure to ionizing radiation. By the 1930s, after a number of cases of bone necrosis and death of radium treatment enthusiasts, radium-containing medicinal products had been largely removed from the market ( radioactive quackery).
Radiation protection
Only a year after Röntgen's discovery of X rays, the American engineer Wolfram Fuchs (1896) gave what is probably the first protection advice, but it was not until 1925 that the first International Congress of Radiology (ICR) was held and considered establishing international protection standards. The effects of radiation on genes, including the effect of cancer risk, were recognized much later. In 1927,Hermann Joseph Muller
Hermann Joseph Muller (December 21, 1890 – April 5, 1967) was an American geneticist, educator, and Nobel laureate best known for his work on the physiological and genetic effects of radiation ( mutagenesis), as well as his outspoken politica ...
published research showing genetic effects and, in 1946, was awarded the Nobel Prize in Physiology or Medicine
The Nobel Prize in Physiology or Medicine is awarded yearly by the Nobel Assembly at the Karolinska Institute for outstanding discoveries in physiology or medicine. The Nobel Prize is not a single prize, but five separate prizes that, accordi ...
for his findings.
The second ICR was held in Stockholm in 1928 and proposed the adoption of the röntgen unit, and the International X-ray and Radium Protection Committee
The International Commission on Radiological Protection (ICRP) is an independent, international, non-governmental organization, with the mission to protect people, animals, and the environment from the harmful effects of ionising radiation. Its r ...
(IXRPC) was formed. Rolf Sievert
Rolf Maximilian Sievert (; 6 May 1896 – 3 October 1966) was a Swedish medical physicist whose major contribution was in the study of the biological effects of ionizing radiation.
Sievert was born in Stockholm, Sweden. His parents were ...
was named Chairman, but a driving force was George Kaye of the British National Physical Laboratory. The committee met in 1931, 1934, and 1937.
After World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the World War II by country, vast majority of the world's countries—including all of the great power ...
, the increased range and quantity of radioactive substances being handled as a result of military and civil nuclear programs led to large groups of occupational workers and the public being potentially exposed to harmful levels of ionising radiation. This was considered at the first post-war ICR convened in London in 1950, when the present International Commission on Radiological Protection (ICRP) was born.
Since then the ICRP has developed the present international system of radiation protection, covering all aspects of radiation hazards.
In 2020, Hauptmann and other 15 international researchers of eight nations, among which: Institutes of Biostatistics, Registry Research, Centers of Cancer Epidemiology, Radiation Epidemiology, and then also U.S. National Cancer Institute (NCI), International Agency for Research on Cancer (IARC) and Radiation Effects Research Foundation of Hiroshima studied definitively through meta-analysis the damage resulting from the "low doses" that have afflicted the populations of survivors of the atomic bombings of Hiroshima and Nagasaki
The United States detonated two atomic bombs over the Japanese cities of Hiroshima and Nagasaki on 6 and 9 August 1945, respectively. The two bombings killed between 129,000 and 226,000 people, most of whom were civilians, and remain the onl ...
and also in numerous accidents of nuclear plants that have occurred in the world. These scientists reported, in JNCI Monographs: Epidemiological Studies of Low Dose Ionizing Radiation and Cancer Risk, that the new epidemiological studies directly support excess cancer risks from low-dose ionizing radiation. In 2021, Italian researcher Venturi reported the first correlations between radio-caesium and pancreatic cancer with the role of caesium in biology and in pancreatitis and in diabetes of pancreatic origin.
Units
 The
The International System of Units
The International System of Units, known by the international abbreviation SI in all languages and sometimes pleonastically as the SI system, is the modern form of the metric system and the world's most widely used system of measurement. ...
(SI) unit of radioactive activity is the becquerel (Bq), named in honor of the scientist Henri Becquerel. One Bq is defined as one transformation (or decay or disintegration) per second.
An older unit of radioactivity is the curie
In computing, a CURIE (or ''Compact URI'') defines a generic, abbreviated syntax for expressing Uniform Resource Identifiers (URIs). It is an abbreviated URI expressed in a compact syntax, and may be found in both XML and non-XML grammars. A CURI ...
, Ci, which was originally defined as "the quantity or mass of radium emanation
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather t ...
in equilibrium with one gram of radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rat ...
(element)". Today, the curie is defined as disintegrations per second, so that 1 curie
In computing, a CURIE (or ''Compact URI'') defines a generic, abbreviated syntax for expressing Uniform Resource Identifiers (URIs). It is an abbreviated URI expressed in a compact syntax, and may be found in both XML and non-XML grammars. A CURI ...
(Ci) = .
For radiological protection purposes, although the United States Nuclear Regulatory Commission permits the use of the unit curie
In computing, a CURIE (or ''Compact URI'') defines a generic, abbreviated syntax for expressing Uniform Resource Identifiers (URIs). It is an abbreviated URI expressed in a compact syntax, and may be found in both XML and non-XML grammars. A CURI ...
alongside SI units, the European Union
The European Union (EU) is a supranational union, supranational political union, political and economic union of Member state of the European Union, member states that are located primarily in Europe, Europe. The union has a total area of ...
European units of measurement directives required that its use for "public health ... purposes" be phased out by 31 December 1985.
The effects of ionizing radiation are often measured in units of gray
Grey (more common in British English) or gray (more common in American English) is an intermediate color between black and white. It is a neutral or achromatic color, meaning literally that it is "without color", because it can be compose ...
for mechanical or sievert for damage to tissue.
Types
magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
could split radioactive emissions into three types of beams. The rays were given the names alpha
Alpha (uppercase , lowercase ; grc, ἄλφα, ''álpha'', or ell, άλφα, álfa) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter aleph , whi ...
, beta
Beta (, ; uppercase , lowercase , or cursive ; grc, βῆτα, bē̂ta or ell, βήτα, víta) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Modern Greek, it represents the voiced labiod ...
, and gamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter r ...
, in increasing order of their ability to penetrate matter. Alpha decay is observed only in heavier elements of atomic number 52 (tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionall ...
) and greater, with the exception of beryllium-8 (which decays to two alpha particles). The other two types of decay are observed in all the elements. Lead, atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
82, is the heaviest element to have any isotopes stable (to the limit of measurement) to radioactive decay. Radioactive decay is seen in all isotopes of all elements of atomic number 83 (bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
) or greater. Bismuth-209, however, is only very slightly radioactive, with a half-life greater than the age of the universe; radioisotopes with extremely long half-lives are considered effectively stable for practical purposes.
alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
s carried a positive charge, beta particles
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β ...
carried a negative charge, and gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
s were neutral. From the magnitude of deflection, it was clear that alpha particles
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
were much more massive than beta particles
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β ...
. Passing alpha particles through a very thin glass window and trapping them in a discharge tube allowed researchers to study the emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. The photon energy of ...
of the captured particles, and ultimately proved that alpha particles are helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
nuclei. Other experiments showed beta radiation, resulting from decay and cathode rays, were high-speed electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
. Likewise, gamma radiation and X-rays were found to be high-energy electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visib ...
.
The relationship between the types of decays also began to be examined: For example, gamma decay was almost always found to be associated with other types of decay, and occurred at about the same time, or afterwards. Gamma decay as a separate phenomenon, with its own half-life (now termed isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ...
), was found in natural radioactivity to be a result of the gamma decay of excited metastable nuclear isomers, which were in turn created from other types of decay.
Although alpha, beta, and gamma radiations were most commonly found, other types of emission were eventually discovered. Shortly after the discovery of the positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. It has an electric charge of +1 '' e'', a spin of 1/2 (the same as the electron), and the same mass as an electron. When a positron collide ...
in cosmic ray products, it was realized that the same process that operates in classical beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
can also produce positrons ( positron emission), along with neutrinos (classical beta decay produces antineutrinos). In a more common analogous process, called electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
, some proton-rich nuclides were found to capture their own atomic electrons instead of emitting positrons, and subsequently, these nuclides emit only a neutrino and a gamma ray from the excited nucleus (and often also Auger electrons and characteristic X-ray Characteristic X-rays are emitted when outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern that is "characteristic" to each element. Characteristic X-rays were discovered by Charles Glover Barkla in 19 ...
s, as a result of the re-ordering of electrons to fill the place of the missing captured electron). These types of decay involve the nuclear capture of electrons or emission of electrons or positrons, and thus acts to move a nucleus toward the ratio of neutrons to protons that has the least energy for a given total number of nucleons. This consequently produces a more stable (lower energy) nucleus.
A hypothetical process of positron capture, analogous to electron capture, is theoretically possible in antimatter atoms, but has not been observed, as complex antimatter atoms beyond antihelium are not experimentally available. Such a decay would require antimatter atoms at least as complex as beryllium-7
Beryllium (4Be) has 11 known isotopes and 3 known isomers, but only one of these isotopes () is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic element, because its other iso ...
, which is the lightest known isotope of normal matter to undergo decay by electron capture.
Shortly after the discovery of the neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
in 1932, Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian (later naturalized American) physicist and the creator of the world's first nuclear reactor, the Chicago Pile-1. He has been called the "architect of the nuclear age" an ...
realized that certain rare beta-decay reactions immediately yield neutrons as an additional decay particle, so called beta-delayed neutron emission. Neutron emission usually happens from nuclei that are in an excited state, such as the excited 17O* produced from the beta decay of 17N. The neutron emission process itself is controlled by the nuclear force and therefore is extremely fast, sometimes referred to as "nearly instantaneous". Isolated proton emission was eventually observed in some elements. It was also found that some heavy elements may undergo spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
into products that vary in composition. In a phenomenon called cluster decay
Cluster decay, also named heavy particle radioactivity or heavy ion radioactivity, is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, but less than a typic ...
, specific combinations of neutrons and protons other than alpha particles (helium nuclei) were found to be spontaneously emitted from atoms.
Other types of radioactive decay were found to emit previously seen particles but via different mechanisms. An example is internal conversion, which results in an initial electron emission, and then often further characteristic X-ray Characteristic X-rays are emitted when outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern that is "characteristic" to each element. Characteristic X-rays were discovered by Charles Glover Barkla in 19 ...
s and Auger electrons emissions, although the internal conversion process involves neither beta nor gamma decay. A neutrino is not emitted, and none of the electron(s) and photon(s) emitted originate in the nucleus, even though the energy to emit all of them does originate there. Internal conversion decay, like isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ...
gamma decay and neutron emission, involves the release of energy by an excited nuclide, without the transmutation of one element into another.
Rare events that involve a combination of two beta-decay-type events happening simultaneously are known (see below). Any decay process that does not violate the conservation of energy or momentum laws (and perhaps other particle conservation laws) is permitted to happen, although not all have been detected. An interesting example discussed in a final section, is bound state beta decay of rhenium-187. In this process, the beta electron-decay of the parent nuclide is not accompanied by beta electron emission, because the beta particle has been captured into the K-shell of the emitting atom. An antineutrino is emitted, as in all negative beta decays.
Radionuclides can undergo a number of different reactions. These are summarized in the following table. A nucleus with mass number ''A'' and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
''Z'' is represented as (''A'', ''Z''). The column "Daughter nucleus" indicates the difference between the new nucleus and the original nucleus. Thus, (''A'' − 1, ''Z'') means that the mass number is one less than before, but the atomic number is the same as before.
If energy circumstances are favorable, a given radionuclide may undergo many competing types of decay, with some atoms decaying by one route, and others decaying by another. An example is copper-64, which has 29 protons, and 35 neutrons, which decays with a half-life of hours. This isotope has one unpaired proton and one unpaired neutron, so either the proton or the neutron can decay to the other particle, which has opposite isospin. This particular nuclide (though not all nuclides in this situation) is more likely to decay through beta plus decay (%) than through electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
(%). The excited energy states resulting from these decays which fail to end in a ground energy state, also produce later internal conversion and gamma decay in almost 0.5% of the time.
More common in heavy nuclides is competition between alpha and beta decay. The daughter nuclides will then normally decay through beta or alpha, respectively, to end up in the same place.
Radioactive decay results in a reduction of summed rest mass
Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a physical body, until the discovery of the atom and particle physics. It was found that different atoms and different ele ...
, once the released energy (the ''disintegration energy'') has escaped in some way. Although decay energy is sometimes defined as associated with the difference between the mass of the parent nuclide products and the mass of the decay products, this is true only of rest mass measurements, where some energy has been removed from the product system. This is true because the decay energy must always carry mass with it, wherever it appears (see mass in special relativity
The word " mass" has two meanings in special relativity: '' invariant mass'' (also called rest mass) is an invariant quantity which is the same for all observers in all reference frames, while the relativistic mass is dependent on the velocity ...
) according to the formula ''E'' = ''mc''2. The decay energy is initially released as the energy of emitted photons plus the kinetic energy of massive emitted particles (that is, particles that have rest mass). If these particles come to thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in ...
with their surroundings and photons are absorbed, then the decay energy is transformed to thermal energy, which retains its mass.
Decay energy, therefore, remains associated with a certain measure of the mass of the decay system, called invariant mass
The invariant mass, rest mass, intrinsic mass, proper mass, or in the case of bound systems simply mass, is the portion of the total mass of an object or system of objects that is independent of the overall motion of the system. More precisely, i ...
, which does not change during the decay, even though the energy of decay is distributed among decay particles. The energy of photons, the kinetic energy of emitted particles, and, later, the thermal energy of the surrounding matter, all contribute to the invariant mass
The invariant mass, rest mass, intrinsic mass, proper mass, or in the case of bound systems simply mass, is the portion of the total mass of an object or system of objects that is independent of the overall motion of the system. More precisely, i ...
of the system. Thus, while the sum of the rest masses of the particles is not conserved in radioactive decay, the ''system'' mass and system invariant mass
The invariant mass, rest mass, intrinsic mass, proper mass, or in the case of bound systems simply mass, is the portion of the total mass of an object or system of objects that is independent of the overall motion of the system. More precisely, i ...
(and also the system total energy) is conserved throughout any decay process. This is a restatement of the equivalent laws of conservation of energy
In physics and chemistry, the law of conservation of energy states that the total energy of an isolated system remains constant; it is said to be ''conserved'' over time. This law, first proposed and tested by Émilie du Châtelet, means tha ...
and conservation of mass.
List of decay modes
Rates
The ''decay rate'', or ''activity'', of a radioactive substance is characterized by the following time-independent parameters: * The ''half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
'', , is the time taken for the activity of a given amount of a radioactive substance to decay to half of its initial value.
* The ''decay constant
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate ...
'', "lambda
Lambda (}, ''lám(b)da'') is the 11th letter of the Greek alphabet, representing the voiced alveolar lateral approximant . In the system of Greek numerals, lambda has a value of 30. Lambda is derived from the Phoenician Lamed . Lambda gave ri ...
", the reciprocal of the mean lifetime (in ), sometimes referred to as simply ''decay rate''.
* The ''mean lifetime
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and ( lambda) is a positive rate ...
'', " tau", the average lifetime (1/ e life) of a radioactive particle before decay.
Although these are constants, they are associated with the statistical behavior of populations of atoms. In consequence, predictions using these constants are less accurate for minuscule samples of atoms.
In principle a half-life, a third-life, or even a (1/√2)-life, can be used in exactly the same way as half-life; but the mean life and half-life have been adopted as standard times associated with exponential decay.
Those parameters can be related to the following time-dependent parameters:
* ''Total activity'', , is the number of decays per unit time of a radioactive sample.
* ''Number of particles'', , is the total number of particles in the sample.
* ''Specific activity'', , is the number of decays per unit time per amount of substance of the sample at time set to zero (). "Amount of substance" can be the mass, volume or moles of the initial sample.
These are related as follows:
:
where ''N''0 is the initial amount of active substance — substance that has the same percentage of unstable particles as when the substance was formed.
Mathematics
Universal law
The mathematics of radioactive decay depend on a key assumption that a nucleus of a radionuclide has no "memory" or way of translating its history into its present behavior. A nucleus does not "age" with the passage of time. Thus, the probability of its breaking down does not increase with time but stays constant, no matter how long the nucleus has existed. This constant probability may differ greatly between one type of nucleus and another, leading to the many different observed decay rates. However, whatever the probability is, it does not change over time. This is in marked contrast to complex objects that do show aging, such as automobiles and humans. These aging systems do have a chance of breakdown per unit of time that increases from the moment they begin their existence. Aggregate processes, like the radioactive decay of a lump of atoms, for which the single-event probability of realization is very small but in which the number of time-slices is so large that there is nevertheless a reasonable rate of events, are modelled by the Poisson distribution, which is discrete. Radioactive decay and nuclear particle reactions are two examples of such aggregate processes. The mathematics of Poisson processes reduce to the law ofexponential decay
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate ...
, which describes the statistical behaviour of a large number of nuclei, rather than one individual nucleus. In the following formalism, the number of nuclei or the nuclei population ''N'', is of course a discrete variable (a natural number
In mathematics, the natural numbers are those numbers used for counting (as in "there are ''six'' coins on the table") and ordering (as in "this is the ''third'' largest city in the country").
Numbers used for counting are called '' cardinal ...
)—but for any physical sample ''N'' is so large that it can be treated as a continuous variable. Differential calculus
In mathematics, differential calculus is a subfield of calculus that studies the rates at which quantities change. It is one of the two traditional divisions of calculus, the other being integral calculus—the study of the area beneath a curve ...
is used to model the behaviour of nuclear decay.
One-decay process
Consider the case of a nuclide that decays into another by some process (emission of other particles, like electron neutrinos andelectron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s e− as in beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
, are irrelevant in what follows). The decay of an unstable nucleus is entirely random in time so it is impossible to predict when a particular atom will decay. However, it is equally likely to decay at any instant in time. Therefore, given a sample of a particular radioisotope, the number of decay events expected to occur in a small interval of time is proportional to the number of atoms present , that is
:
Particular radionuclides decay at different rates, so each has its own decay constant . The expected decay is proportional to an increment of time, :
The negative sign indicates that decreases as time increases, as the decay events follow one after another. The solution to this first-order differential equation
In mathematics, a differential equation is an equation that relates one or more unknown functions and their derivatives. In applications, the functions generally represent physical quantities, the derivatives represent their rates of change, ...
is the function:
:
where is the value of at time = 0, with the decay constant expressed as
We have for all time :
:
where is the constant number of particles throughout the decay process, which is equal to the initial number of nuclides since this is the initial substance.
If the number of non-decayed nuclei is:
:
then the number of nuclei of (i.e. the number of decayed nuclei) is
:
The number of decays observed over a given interval obeys Poisson statistics
In probability theory and statistics, the Poisson distribution is a discrete probability distribution that expresses the probability of a given number of events occurring in a fixed interval of time or space if these events occur with a known c ...
. If the average number of decays is , the probability of a given number of decays is
:
Chain-decay processes
=Chain of two decays
= Now consider the case of a chain of two decays: one nuclide decaying into another by one process, then decaying into another by a second process, i.e. '. The previous equation cannot be applied to the decay chain, but can be generalized as follows. Since decays into , ''then'' decays into , the activity of adds to the total number of nuclides in the present sample, ''before'' those nuclides decay and reduce the number of nuclides leading to the later sample. In other words, the number of second generation nuclei increases as a result of the first generation nuclei decay of , and decreases as a result of its own decay into the third generation nuclei .Introductory Nuclear Physics, K.S. Krane, 1988, John Wiley & Sons Inc, The sum of these two terms gives the law for a decay chain for two nuclides: : The rate of change of , that is , is related to the changes in the amounts of and , can increase as is produced from and decrease as produces . Re-writing using the previous results: The subscripts simply refer to the respective nuclides, i.e. is the number of nuclides of type ; is the initial number of nuclides of type ; is the decay constant for – and similarly for nuclide . Solving this equation for gives: : In the case where is a stable nuclide ( = 0), this equation reduces to the previous solution: : as shown above for one decay. The solution can be found by the integration factor method, where the integrating factor is . This case is perhaps the most useful since it can derive both the one-decay equation (above) and the equation for multi-decay chains (below) more directly.=Chain of any number of decays
= For the general case of any number of consecutive decays in a decay chain, i.e. , where is the number of decays and is a dummy index (), each nuclide population can be found in terms of the previous population. In this case , , ..., . Using the above result in a recursive form: : The general solution to the recursive problem is given by Bateman's equations:Alternative modes
In all of the above examples, the initial nuclide decays into just one product. Consider the case of one initial nuclide that can decay into either of two products, that is ' and ' in parallel. For example, in a sample of potassium-40, 89.3% of the nuclei decay tocalcium-40
Calcium (20Ca) has 26 known isotopes, ranging from 35Ca to 60Ca. There are five stable isotopes (40Ca, 42Ca, 43Ca, 44Ca and 46Ca), plus one isotope ( 48Ca) with such a long half-life that for all practical purposes it can be considered stable. T ...
and 10.7% to argon-40
Argon (18Ar) has 26 known isotopes, from 29Ar to 54Ar and 1 isomer (32mAr), of which three are stable (36Ar, 38Ar, and 40Ar). On the Earth, 40Ar makes up 99.6% of natural argon. The longest-lived radioactive isotopes are 39Ar with a half-life of ...
. We have for all time :
:
which is constant, since the total number of nuclides remains constant. Differentiating with respect to time:
:
defining the ''total decay constant'' in terms of the sum of ''partial decay constants'' and :
:
Solving this equation for :
:
where is the initial number of nuclide A. When measuring the production of one nuclide, one can only observe the total decay constant . The decay constants and determine the probability for the decay to result in products or as follows:
:
:
because the fraction of nuclei decay into while the fraction of nuclei decay into .
Corollaries of laws
The above equations can also be written using quantities related to the number of nuclide particles in a sample; * The activity: . * Theamount of substance
In chemistry, the amount of substance ''n'' in a given sample of matter is defined as the quantity or number of discrete atomic-scale particles in it divided by the Avogadro constant ''N''A. The particles or entities may be molecules, atoms, io ...
: .
* The mass
Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a physical body, until the discovery of the atom and particle physics. It was found that different atoms and different ele ...
: .
where = is the Avogadro constant
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining ...
, is the molar mass of the substance in kg/mol, and the amount of the substance is in moles Moles can refer to:
* Moles de Xert, a mountain range in the Baix Maestrat comarca, Valencian Community, Spain
*The Moles (Australian band)
*The Moles, alter ego of Scottish band Simon Dupree and the Big Sound
People
* Abraham Moles, French engin ...
.
Decay timing: definitions and relations
Time constant and mean-life
For the one-decay solution ': : the equation indicates that thedecay constant
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate ...
has units of , and can thus also be represented as 1/, where is a characteristic time of the process called the '' time constant''.
In a radioactive decay process, this time constant is also the mean lifetime
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and ( lambda) is a positive rate ...
for decaying atoms. Each atom "lives" for a finite amount of time before it decays, and it may be shown that this mean lifetime is the arithmetic mean
In mathematics and statistics, the arithmetic mean ( ) or arithmetic average, or just the '' mean'' or the ''average'' (when the context is clear), is the sum of a collection of numbers divided by the count of numbers in the collection. The co ...
of all the atoms' lifetimes, and that it is , which again is related to the decay constant as follows:
:
This form is also true for two-decay processes simultaneously ', inserting the equivalent values of decay constants (as given above)
:
into the decay solution leads to:
:

Half-life
A more commonly used parameter is thehalf-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
. Given a sample of a particular radionuclide, the half-life is the time taken for half the radionuclide's atoms to decay. For the case of one-decay nuclear reactions:
:
the half-life is related to the decay constant as follows: set and = to obtain
:
This relationship between the half-life and the decay constant shows that highly radioactive substances are quickly spent, while those that radiate weakly endure longer. Half-lives of known radionuclides vary by almost 54 orders of magnitude, from more than years ( sec) for the very nearly stable nuclide 128Te, to seconds for the highly unstable nuclide 5H.
The factor of in the above relations results from the fact that the concept of "half-life" is merely a way of selecting a different base other than the natural base for the lifetime expression. The time constant is the -life, the time until only 1/''e'' remains, about 36.8%, rather than the 50% in the half-life of a radionuclide. Thus, is longer than . The following equation can be shown to be valid:
:
Since radioactive decay is exponential with a constant probability, each process could as easily be described with a different constant time period that (for example) gave its "(1/3)-life" (how long until only 1/3 is left) or "(1/10)-life" (a time period until only 10% is left), and so on. Thus, the choice of and for marker-times, are only for convenience, and from convention. They reflect a fundamental principle only in so much as they show that the ''same proportion'' of a given radioactive substance will decay, during any time-period that one chooses.
Mathematically, the life for the above situation would be found in the same way as aboveby setting ', and substituting into the decay solution to obtain
:
Example for carbon-14
Carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and co ...
has a half-life of years and a decay rate of 14 disintegrations per minute (dpm) per gram of natural carbon.
If an artifact is found to have radioactivity of 4 dpm per gram of its present C, we can find the approximate age of the object using the above equation:
:
where:
:
Changing rates
The radioactive decay modes ofelectron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
and internal conversion are known to be slightly sensitive to chemical and environmental effects that change the electronic structure of the atom, which in turn affects the presence of 1s and 2s electrons that participate in the decay process. A small number of nuclides are affected. For example, chemical bonds can affect the rate of electron capture to a small degree (in general, less than 1%) depending on the proximity of electrons to the nucleus. In 7Be, a difference of 0.9% has been observed between half-lives in metallic and insulating environments. This relatively large effect is because beryllium is a small atom whose valence electrons are in 2s atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any ...
s, which are subject to electron capture in 7Be because (like all s atomic orbitals in all atoms) they naturally penetrate into the nucleus.
In 1992, Jung et al. of the Darmstadt Heavy-Ion Research group observed an accelerated β− decay of 163Dy66+. Although neutral 163Dy is a stable isotope, the fully ionized 163Dy66+ undergoes β− decay into the K and L shells to 163Ho66+ with a half-life of 47 days.
Rhenium-187 is another spectacular example. 187Re normally undergoes beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
to 187Os with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 41.6 × 109 years, but studies using fully ionised 187 Re atoms (bare nuclei) have found that this can decrease to only 32.9 years. This is attributed to " bound-state β− decay" of the fully ionised atom – the electron is emitted into the "K-shell" (1s atomic orbital), which cannot occur for neutral atoms in which all low-lying bound states are occupied.
 A number of experiments have found that decay rates of other modes of artificial and naturally occurring radioisotopes are, to a high degree of precision, unaffected by external conditions such as temperature, pressure, the chemical environment, and electric, magnetic, or gravitational fields. Comparison of laboratory experiments over the last century, studies of the Oklo natural nuclear reactor (which exemplified the effects of thermal neutrons on nuclear decay), and astrophysical observations of the luminosity decays of distant supernovae (which occurred far away so the light has taken a great deal of time to reach us), for example, strongly indicate that unperturbed decay rates have been constant (at least to within the limitations of small experimental errors) as a function of time as well.
Recent results suggest the possibility that decay rates might have a weak dependence on environmental factors. It has been suggested that measurements of decay rates of
A number of experiments have found that decay rates of other modes of artificial and naturally occurring radioisotopes are, to a high degree of precision, unaffected by external conditions such as temperature, pressure, the chemical environment, and electric, magnetic, or gravitational fields. Comparison of laboratory experiments over the last century, studies of the Oklo natural nuclear reactor (which exemplified the effects of thermal neutrons on nuclear decay), and astrophysical observations of the luminosity decays of distant supernovae (which occurred far away so the light has taken a great deal of time to reach us), for example, strongly indicate that unperturbed decay rates have been constant (at least to within the limitations of small experimental errors) as a function of time as well.
Recent results suggest the possibility that decay rates might have a weak dependence on environmental factors. It has been suggested that measurements of decay rates of silicon-32
Silicon (14Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. 28Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable. The longest-lived radioisotope is 32Si, which is produced by cosmic ray spallatio ...
, manganese-54
Naturally occurring manganese (25Mn) is composed of one stable isotope, 55Mn. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3.7 million years, 54Mn with a half-life of 312.3 days, and 52Mn with a hal ...
, and radium-226 exhibit small seasonal variations (of the order of 0.1%). However, such measurements are highly susceptible to systematic errors, and a subsequent paper has found no evidence for such correlations in seven other isotopes (22Na, 44Ti, 108Ag, 121Sn, 133Ba, 241Am, 238Pu), and sets upper limits on the size of any such effects. The decay of radon-222 was once reported to exhibit large 4% peak-to-peak seasonal variations (see plot), which were proposed to be related to either solar flare activity or the distance from the Sun, but detailed analysis of the experiment's design flaws, along with comparisons to other, much more stringent and systematically controlled, experiments refute this claim.
GSI anomaly
An unexpected series of experimental results for the rate of decay of heavy highly charged radioactive ions circulating in a storage ring has provoked theoretical activity in an effort to find a convincing explanation. The rates ofweak
Weak may refer to:
Songs
* "Weak" (AJR song), 2016
* "Weak" (Melanie C song), 2011
* "Weak" (SWV song), 1993
* "Weak" (Skunk Anansie song), 1995
* "Weak", a song by Seether from '' Seether: 2002-2013''
Television episodes
* "Weak" (''Fear t ...
decay of two radioactive species with half lives of about 40 s and 200 s are found to have a significant oscillatory
Oscillation is the repetitive or periodic variation, typically in time, of some measure about a central value (often a point of equilibrium) or between two or more different states. Familiar examples of oscillation include a swinging pendulum ...
modulation
In electronics and telecommunications, modulation is the process of varying one or more properties of a periodic waveform, called the '' carrier signal'', with a separate signal called the ''modulation signal'' that typically contains informat ...
, with a period of about 7 s.
The observed phenomenon is known as the GSI anomaly
One of the experimental facilities at the German laboratory GSI Helmholtz Centre for Heavy Ion Research in Darmstadt is an Experimental Storage Ring (ESR) with electron cooling in which large numbers of highly charged radioactive ions can be stor ...
, as the storage ring is a facility at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt
Darmstadt () is a city in the state of Hesse in Germany, located in the southern part of the Rhine-Main-Area (Frankfurt Metropolitan Region). Darmstadt has around 160,000 inhabitants, making it the fourth largest city in the state of Hesse ...
, Germany
Germany,, officially the Federal Republic of Germany, is a country in Central Europe. It is the second most populous country in Europe after Russia, and the most populous member state of the European Union. Germany is situated betwee ...
. As the decay process produces an electron neutrino, some of the proposed explanations for the observed rate oscillation invoke neutrino properties. Initial ideas related to flavour oscillation met with skepticism. A more recent proposal involves mass differences between neutrino mass eigenstates.
Theoretical basis
Theneutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s and proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s that constitute nuclei, as well as other particles that approach close enough to them, are governed by several interactions. The nuclear force (also known as ''residual'' strong force), not observed at the familiar macroscopic scale, is the most powerful force over subatomic distances. The electrostatic force is almost always significant, and, in the case of beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
, the weak nuclear force
In nuclear physics and particle physics, the weak interaction, which is also often called the weak force or weak nuclear force, is one of the four known fundamental interactions, with the others being electromagnetism, the strong interacti ...
is also involved.
The combined effects of these forces produces a number of different phenomena in which energy may be released by rearrangement of particles in the nucleus, or else the change of one type of particle into others. These rearrangements and transformations may be hindered energetically so that they do not occur immediately. In certain cases, random quantum vacuum fluctuations are theorized to promote relaxation to a lower energy state (the "decay") in a phenomenon known as quantum tunneling. Radioactive decay half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of nuclides has been measured over timescales of 54 orders of magnitude, from seconds (for hydrogen-5
Hydrogen (1H) has three naturally occurring isotopes, sometimes denoted , , and . and are stable, while has a half-life of years. Heavier isotopes also exist, all of which are synthetic and have a half-life of less than one zeptosecond (10� ...
) to seconds (for tellurium-128
There are 39 known isotopes and 17 nuclear isomers of tellurium (52Te), with atomic masses that range from 104 to 142. These are listed in the table below.
Naturally-occurring tellurium on Earth consists of eight isotopes. Two of these have been ...
). The limits of these timescales are set by the sensitivity of instrumentation only, and there are no known natural limits to how brief or long a decay half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
for radioactive decay of a radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
may be.
The decay process, like all hindered energy transformations, may be analogized by a snowfield on a mountain. While friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction:
*Dry friction is a force that opposes the relative lateral motion of ...
between the ice crystals may be supporting the snow's weight, the system is inherently unstable with regard to a state of lower potential energy. A disturbance would thus facilitate the path to a state of greater entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
; the system will move towards the ground state, producing heat, and the total energy will be distributable over a larger number of quantum states
In quantum physics, a quantum state is a mathematical entity that provides a probability distribution for the outcomes of each possible measurement on a system. Knowledge of the quantum state together with the rules for the system's evolution in ...
thus resulting in an avalanche
An avalanche is a rapid flow of snow down a slope, such as a hill or mountain.
Avalanches can be set off spontaneously, by such factors as increased precipitation or snowpack weakening, or by external means such as humans, animals, and ea ...
. The ''total'' energy does not change in this process, but, because of the second law of thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unle ...
, avalanches have only been observed in one direction and that is toward the "ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
" — the state with the largest number of ways in which the available energy could be distributed.
Such a collapse (a gamma-ray ''decay event'') requires a specific activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
. For a snow avalanche, this energy comes as a disturbance from outside the system, although such disturbances can be arbitrarily small. In the case of an excited atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron ...
decaying by gamma radiation in a spontaneous emission
Spontaneous emission is the process in which a quantum mechanical system (such as a molecule, an atom or a subatomic particle) transits from an excited energy state to a lower energy state (e.g., its ground state) and emits a quantized amount ...
of electromagnetic radiation, the arbitrarily small disturbance comes from quantum vacuum fluctuations.
A radioactive nucleus (or any excited system in quantum mechanics) is unstable, and can, thus, ''spontaneously'' stabilize to a less-excited system. The resulting transformation alters the structure of the nucleus and results in the emission of either a photon or a high-velocity particle that has mass (such as an electron, alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
, or other type).
Occurrence and applications
According to theBig Bang theory
The Big Bang event is a physical theory that describes how the universe expanded from an initial state of high density and temperature. Various cosmological models of the Big Bang explain the evolution of the observable universe from the ...
, stable isotopes of the lightest three elements ( H, He, and traces of Li) were produced very shortly after the emergence of the universe, in a process called Big Bang nucleosynthesis. These lightest stable nuclides (including deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one ...
) survive to today, but any radioactive isotopes of the light elements produced in the Big Bang (such as tritium) have long since decayed. Isotopes of elements heavier than boron were not produced at all in the Big Bang, and these first five elements do not have any long-lived radioisotopes. Thus, all radioactive nuclei are, therefore, relatively young with respect to the birth of the universe, having formed later in various other types of nucleosynthesis in star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked ...
s (in particular, supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or whe ...
e), and also during ongoing interactions between stable isotopes and energetic particles. For example, carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and co ...
, a radioactive nuclide with a half-life of only years, is constantly produced in Earth's upper atmosphere due to interactions between cosmic rays and nitrogen.
Nuclides that are produced by radioactive decay are called radiogenic nuclides, whether they themselves are stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
or not. There exist stable radiogenic nuclides that were formed from short-lived extinct radionuclides in the early Solar System. The extra presence of these stable radiogenic nuclides (such as xenon-129 from extinct iodine-129) against the background of primordial stable nuclides can be inferred by various means.
Radioactive decay has been put to use in the technique of radioisotopic labeling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific ...
, which is used to track the passage of a chemical substance through a complex system (such as a living organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells ( cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and fu ...
). A sample of the substance is synthesized with a high concentration of unstable atoms. The presence of the substance in one or another part of the system is determined by detecting the locations of decay events.
On the premise that radioactive decay is truly random
In common usage, randomness is the apparent or actual lack of pattern or predictability in events. A random sequence of events, symbols or steps often has no order and does not follow an intelligible pattern or combination. Individual ran ...
(rather than merely chaotic
Chaotic was originally a Danish trading card game. It expanded to an online game in America which then became a television program based on the game. The program was able to be seen on 4Kids TV (Fox affiliates, nationwide), Jetix, The CW4Kid ...
), it has been used in hardware random-number generator
In computing, a hardware random number generator (HRNG) or true random number generator (TRNG) is a device that generates random numbers from a physical process, rather than by means of an algorithm. Such devices are often based on microscop ...
s. Because the process is not thought to vary significantly in mechanism over time, it is also a valuable tool in estimating the absolute ages of certain materials. For geological materials, the radioisotopes and some of their decay products become trapped when a rock solidifies, and can then later be used (subject to many well-known qualifications) to estimate the date of the solidification. These include checking the results of several simultaneous processes and their products against each other, within the same sample. In a similar fashion, and also subject to qualification, the rate of formation of carbon-14 in various eras, the date of formation of organic matter within a certain period related to the isotope's half-life may be estimated, because the carbon-14 becomes trapped when the organic matter grows and incorporates the new carbon-14 from the air. Thereafter, the amount of carbon-14 in organic matter decreases according to decay processes that may also be independently cross-checked by other means (such as checking the carbon-14 in individual tree rings, for example).
Szilard–Chalmers effect
The Szilard–Chalmers effect is the breaking of a chemical bond as a result of a kinetic energy imparted from radioactive decay. It operates by the absorption of neutrons by an atom and subsequent emission ofgamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
s, often with significant amounts of kinetic energy. This kinetic energy, by Newton's third law, pushes back on the decaying atom, which causes it to move with enough speed to break a chemical bond. This effect can be used to separate isotopes by chemical means.
The Szilard–Chalmers effect was discovered in 1934 by Leó Szilárd and Thomas A. Chalmers. They observed that after bombardment by neutrons, the breaking of a bond in liquid ethyl iodide allowed radioactive iodine to be removed.
Origins of radioactive nuclides
Radioactiveprimordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
s found in the Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's sur ...
are residues from ancient supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or whe ...
explosions that occurred before the formation of the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
. They are the fraction of radionuclides that survived from that time, through the formation of the primordial solar nebula
A nebula ('cloud' or 'fog' in Latin; pl. nebulae, nebulæ or nebulas) is a distinct luminescent part of interstellar medium, which can consist of ionized, neutral or molecular hydrogen and also cosmic dust. Nebulae are often star-forming regio ...
, through planet accretion
Accretion may refer to:
Science
* Accretion (astrophysics), the formation of planets and other bodies by collection of material through gravity
* Accretion (meteorology), the process by which water vapor in clouds forms water droplets around nucl ...
, and up to the present time. The naturally occurring short-lived radiogenic radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
s found in today's rocks, are the daughters of those radioactive primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
s. Another minor source of naturally occurring radioactive nuclides are cosmogenic nuclides, that are formed by cosmic ray bombardment of material in the Earth's atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A ...
or crust. The decay of the radionuclides in rocks of the Earth's mantle
A mantle is a piece of clothing, a type of cloak. Several other meanings are derived from that.
Mantle may refer to:
*Mantle (clothing), a cloak-like garment worn mainly by women as fashionable outerwear
**Mantle (vesture), an Eastern Orthodox ve ...
and crust contribute significantly to Earth's internal heat budget.
Decay chains and multiple modes
The daughter nuclide of a decay event may also be unstable (radioactive). In this case, it too will decay, producing radiation. The resulting second daughter nuclide may also be radioactive. This can lead to a sequence of several decay events called a '' decay chain'' (see this article for specific details of important natural decay chains). Eventually, a stable nuclide is produced. Any decay daughters that are the result of an alpha decay will also result in helium atoms being created. An example is the natural decay chain of 238U:
* Uranium-238 decays, through alpha-emission, with a
An example is the natural decay chain of 238U:
* Uranium-238 decays, through alpha-emission, with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of billion years to thorium-234
Thorium (90Th) has seven naturally occurring isotopes but none are stable. One isotope, 232Th, is ''relatively'' stable, with a half-life of 1.405×1010 years, considerably longer than the age of the Earth, and even slightly longer than the gen ...
* which decays, through beta-emission, with a half-life of days to protactinium-234m
* which decays, through beta-emission, with a half-life of minutes to uranium-234
Uranium-234 (234U or U-234) is an isotope of uranium. In natural uranium and in uranium ore, 234U occurs as an indirect decay product of uranium-238, but it makes up only 0.0055% (55 parts per million) of the raw uranium because its half-life ...
* which decays, through alpha-emission, with a half-life of thousand years to thorium-230
* which decays, through alpha-emission, with a half-life of thousand years to radium-226
* which decays, through alpha-emission, with a half-life of thousand years to radon-222
* which decays, through alpha-emission, with a half-life of days to polonium-218
* which decays, through alpha-emission, with a half-life of minutes to lead-214
Lead (82Pb) has four stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains: the urani ...
* which decays, through beta-emission, with a half-life of minutes to bismuth-214
Bismuth (83Bi) has 41 known isotopes, ranging from 184Bi to 224Bi. Bismuth has no stable isotopes, but does have one very long-lived isotope; thus, the standard atomic weight can be given as . Although bismuth-209 is now known to be unstable, it h ...
* which decays, through beta-emission, with a half-life of minutes to polonium-214
Polonium (84Po) has 42 isotopes, all of which are radioactive, with between 186 and 227 nucleons. 210Po with a half-life of 138.376 days has the longest half-life of naturally occurring polonium. 209Po, with a half-life of 125.2 years, has the lo ...
* which decays, through alpha-emission, with a half-life of microseconds to lead-210
* which decays, through beta-emission, with a half-life of years to bismuth-210
* which decays, through beta-emission, with a half-life of days to polonium-210
Polonium-210 (210Po, Po-210, historically radium F) is an isotope of polonium. It undergoes alpha decay to stable 206Pb with a half-life of 138.376 days (about months), the longest half-life of all naturally occurring polonium isotopes. First ...
* which decays, through alpha-emission, with a half-life of days to lead-206, which is a stable nuclide.
Some radionuclides may have several different paths of decay. For example, % of bismuth-212
Bismuth (83Bi) has 41 known isotopes, ranging from 184Bi to 224Bi. Bismuth has no stable isotopes, but does have one very long-lived isotope; thus, the standard atomic weight can be given as . Although bismuth-209 is now known to be unstable, it ...
decays, through alpha-emission, to thallium-208 while % of bismuth-212
Bismuth (83Bi) has 41 known isotopes, ranging from 184Bi to 224Bi. Bismuth has no stable isotopes, but does have one very long-lived isotope; thus, the standard atomic weight can be given as . Although bismuth-209 is now known to be unstable, it ...
decays, through beta-emission, to polonium-212
Polonium (84Po) has 42 isotopes, all of which are radioactive, with between 186 and 227 nucleons. 210Po with a half-life of 138.376 days has the longest half-life of naturally occurring polonium. 209Po, with a half-life of 125.2 years, has the lo ...
. Both thallium-208 and polonium-212
Polonium (84Po) has 42 isotopes, all of which are radioactive, with between 186 and 227 nucleons. 210Po with a half-life of 138.376 days has the longest half-life of naturally occurring polonium. 209Po, with a half-life of 125.2 years, has the lo ...
are radioactive daughter products of bismuth-212
Bismuth (83Bi) has 41 known isotopes, ranging from 184Bi to 224Bi. Bismuth has no stable isotopes, but does have one very long-lived isotope; thus, the standard atomic weight can be given as . Although bismuth-209 is now known to be unstable, it ...
, and both decay directly to stable lead-208
Lead (82Pb) has four stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains: the urani ...
.
Hazard warning signs
hazard symbol
Hazard symbols or warning symbols are recognisable symbols designed to warn about hazardous or dangerous materials, locations, or objects, including electric currents, poisons, and radioactivity. The use of hazard symbols is often regulated ...
intended for IAEA Category 1, 2 and 3 sources defined as dangerous sources capable of death or serious injury/ref> File:Dangclass7.svg, The dangerous goods transport classification sign for radioactive materials
See also
* Actinides in the environment *Background radiation
Background radiation is a measure of the level of ionizing radiation present in the environment at a particular location which is not due to deliberate introduction of radiation sources.
Background radiation originates from a variety of source ...
* Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two n ...
* Crimes involving radioactive substances
* Decay correction
Decay correction is a method of estimating the amount of radioactive decay at some set time before it was actually measured.
Example of use
Researchers often want to measure, say, medical compounds in the bodies of animals. It's hard to measure t ...
* Fallout shelter
* Geiger counter
A Geiger counter (also known as a Geiger–Müller counter) is an electronic instrument used for detecting and measuring ionizing radiation. It is widely used in applications such as radiation dosimetry, radiological protection, experimental p ...
* Induced radioactivity
* Lists of nuclear disasters and radioactive incidents
* National Council on Radiation Protection and Measurements
* Nuclear engineering
Nuclear engineering is the branch of engineering concerned with the application of breaking down atomic nuclei ( fission) or of combining atomic nuclei ( fusion), or with the application of other sub-atomic processes based on the principles of n ...
* Nuclear pharmacy
Nuclear pharmacy, also known as radiopharmacy, involves preparation of radioactive materials for patient administration that will be used to diagnose and treat specific diseases in nuclear medicine. It generally involves the practice of combining ...
* Nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies t ...
* Nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced b ...
* Particle decay
* Poisson process
* Radiation therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Rad ...
* Radioactive contamination
* Radioactivity in biology
* Radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares ...
* Transient equilibrium In nuclear physics, transient equilibrium is a situation in which equilibrium is reached by a parent-daughter radioactive isotope pair where the half-life of the daughter is shorter than the half-life of the parent. Contrary to secular equilibrium, ...
Notes
References
Inline
General
"Radioactivity"
Encyclopædia Britannica. 2006.
Encyclopædia Britannica Online
An encyclopedia (American English) or encyclopædia (British English) is a reference work or compendium providing summaries of knowledge either general or special to a particular field or discipline. Encyclopedias are divided into article ...
. December 18, 2006
* Radio-activity by Ernest Rutherford Phd, Encyclopædia Britannica Eleventh Edition
The ''Encyclopædia Britannica'' Eleventh Edition (1910–1911) is a 29-volume reference work, an edition of the '' Encyclopædia Britannica''. It was developed during the encyclopaedia's transition from a British to an American publication. S ...
External links
The Lund/LBNL Nuclear Data Search
– Contains tabulated information on radioactive decay types and energies.
Nomenclature of nuclear chemistry
The Live Chart of Nuclides – IAEA
Interactive Chart of Nuclides
Health Physics Society Public Education Website
*
Annotated bibliography for radioactivity from the Alsos Digital Library for Nuclear Issues
by Wolfgang Bauer
by David M. Harrison * "Henri Becquerel: The Discovery of Radioactivity", Becquerel's 1896 articles online and analyzed on
BibNum
'
lick 'à télécharger' for English version
Lick may refer to:
* Licking, the action of passing the tongue over a surface
Places
* Lick (crater), a crater on the Moon named after James Lick
* 1951 Lick, an asteroid named after James Lick
* Lick Township, Jackson County, Ohio, United Sta ...
/small>.
* "Radioactive change", Rutherford & Soddy article (1903), online and analyzed on Bibnum
'
lick 'à télécharger' for English version
Lick may refer to:
* Licking, the action of passing the tongue over a surface
Places
* Lick (crater), a crater on the Moon named after James Lick
* 1951 Lick, an asteroid named after James Lick
* Lick Township, Jackson County, Ohio, United Sta ...
/small>.
{{DEFAULTSORT:Radioactive Decay
Exponentials
Poisson point processes