Protein structure on:
[Wikipedia]
[Google]
[Amazon]
Protein structure is the three-dimensional arrangement of atoms in an

 Secondary structure refers to highly regular local sub-structures on the actual polypeptide backbone chain. Two main types of secondary structure, the α-helix and the β-strand or β-sheets, were suggested in 1951 by Linus Pauling et al. These secondary structures are defined by patterns of hydrogen bonds between the main-chain peptide groups. They have a regular geometry, being constrained to specific values of the dihedral angles ψ and φ on the
Secondary structure refers to highly regular local sub-structures on the actual polypeptide backbone chain. Two main types of secondary structure, the α-helix and the β-strand or β-sheets, were suggested in 1951 by Linus Pauling et al. These secondary structures are defined by patterns of hydrogen bonds between the main-chain peptide groups. They have a regular geometry, being constrained to specific values of the dihedral angles ψ and φ on the
 Proteins are frequently described as consisting of several structural units. These units include domains, motifs, and folds. Despite the fact that there are about 100,000 different proteins expressed in
Proteins are frequently described as consisting of several structural units. These units include domains, motifs, and folds. Despite the fact that there are about 100,000 different proteins expressed in
 Proteins are often thought of as relatively stable tertiary structures that experience conformational changes after being affected by interactions with other proteins or as a part of enzymatic activity. However, proteins may have varying degrees of stability, and some of the less stable variants are
Proteins are often thought of as relatively stable tertiary structures that experience conformational changes after being affected by interactions with other proteins or as a part of enzymatic activity. However, proteins may have varying degrees of stability, and some of the less stable variants are
Protein Ensemble Database
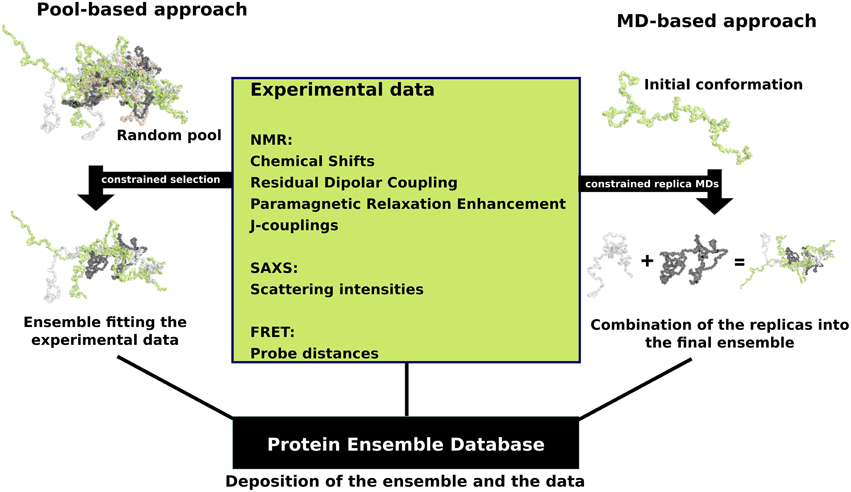
that fall into two general methodologies – pool and molecular dynamics (MD) approaches (diagrammed in the figure). The pool based approach uses the protein’s amino acid sequence to create a massive pool of random conformations. This pool is then subjected to more computational processing that creates a set of theoretical parameters for each conformation based on the structure. Conformational subsets from this pool whose average theoretical parameters closely match known experimental data for this protein are selected. The alternative molecular dynamics approach takes multiple random conformations at a time and subjects all of them to experimental data. Here the experimental data is serving as limitations to be placed on the conformations (e.g. known distances between atoms). Only conformations that manage to remain within the limits set by the experimental data are accepted. This approach often applies large amounts of experimental data to the conformations which is a very computationally demanding task. The conformational ensembles were generated for a number of highly dynamic and partially unfolded proteins, such as
 Around 90% of the protein structures available in the
Around 90% of the protein structures available in the
50 Years of Protein Structure Determination Timeline - HTML Version - National Institute of General Medical Sciences
at
amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
-chain molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
. Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s are polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s specifically polypeptides formed from sequences of amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s, the monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a ...
s, in which the amino acids lose one water molecule per reaction
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
* Reaction (physics), as defined by Newton's third law
*Chain reaction (disambiguation).
Biology and m ...
in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
, ionic interactions, Van der Waals forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
, and hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensional structure. This is the topic of the scientific field of structural biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every le ...
, which employs techniques such as X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
, NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
, cryo electron microscopy (cryo-EM) and dual polarisation interferometry
Dual-polarization interferometry (DPI) is an analytical technique that probes molecular layers adsorbed to the surface of a waveguide using the evanescent wave of a laser beam. It is used to measure the conformational change in proteins, or othe ...
to determine the structure of proteins.
Protein structures range in size from tens to several thousand amino acids. By physical size, proteins are classified as nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
s, between 1–100 nm. Very large protein complexes
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multienzyme complexes, in which multiple catalytic domains are found in a single polypeptide chain.
Protein ...
can be formed from protein subunits. For example, many thousands of actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ov ...
molecules assemble into a microfilament
Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other pr ...
.
A protein usually undergoes reversible structural changes in performing its biological function. The alternative structures of the same protein are referred to as different conformations, and transitions between them are called conformational changes.
Levels of protein structure
There are four distinct levels of protein structure.
Primary structure
The primary structure of a protein refers to the sequence ofamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s in the polypeptide chain. The primary structure is held together by peptide bonds
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
that are made during the process of protein biosynthesis. The two ends of the polypeptide chain
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
are referred to as the carboxyl terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
(C-terminus) and the amino terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
(N-terminus) based on the nature of the free group on each extremity. Counting of residues always starts at the N-terminal end (NH2-group), which is the end where the amino group is not involved in a peptide bond. The primary structure of a protein is determined by the gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
corresponding to the protein. A specific sequence of nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecule ...
s in DNA is transcribed into mRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the ...
, which is read by the ribosome in a process called translation
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
. The sequence of amino acids in insulin was discovered by Frederick Sanger, establishing that proteins have defining amino acid sequences. The sequence of a protein is unique to that protein, and defines the structure and function of the protein. The sequence of a protein can be determined by methods such as Edman degradation
Edman degradation, developed by Pehr Edman, is a method of sequencing amino acids in a peptide. In this method, the amino-terminal residue is labeled and cleaved from the peptide without disrupting the peptide bonds between other amino acid residu ...
or tandem mass spectrometry
Tandem mass spectrometry, also known as MS/MS or MS2, is a technique in instrumental analysis where two or more mass analyzers are coupled together using an additional reaction step to increase their abilities to analyse chemical samples. A com ...
. Often, however, it is read directly from the sequence of the gene using the genetic code
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
. It is strictly recommended to use the words "amino acid residues" when discussing proteins because when a peptide bond is formed, a water molecule is lost, and therefore proteins are made up of amino acid residues. Post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribos ...
s such as phosphorylations and glycosylations are usually also considered a part of the primary structure, and cannot be read from the gene. For example, insulin is composed of 51 amino acids in 2 chains. One chain has 31 amino acids, and the other has 20 amino acids.
Secondary structure
 Secondary structure refers to highly regular local sub-structures on the actual polypeptide backbone chain. Two main types of secondary structure, the α-helix and the β-strand or β-sheets, were suggested in 1951 by Linus Pauling et al. These secondary structures are defined by patterns of hydrogen bonds between the main-chain peptide groups. They have a regular geometry, being constrained to specific values of the dihedral angles ψ and φ on the
Secondary structure refers to highly regular local sub-structures on the actual polypeptide backbone chain. Two main types of secondary structure, the α-helix and the β-strand or β-sheets, were suggested in 1951 by Linus Pauling et al. These secondary structures are defined by patterns of hydrogen bonds between the main-chain peptide groups. They have a regular geometry, being constrained to specific values of the dihedral angles ψ and φ on the Ramachandran plot
In biochemistry, a Ramachandran plot (also known as a Rama plot, a Ramachandran diagram or a �,ψplot), originally developed in 1963 by G. N. Ramachandran, C. Ramakrishnan, and V. Sasisekharan, is a way to visualize energetically allowed regions ...
. Both the α-helix and the β-sheet represent a way of saturating all the hydrogen bond donors and acceptors in the peptide backbone. Some parts of the protein are ordered but do not form any regular structures. They should not be confused with random coil
In polymer chemistry, a random coil is a conformation of polymers where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the ch ...
, an unfolded polypeptide chain lacking any fixed three-dimensional structure. Several sequential secondary structures may form a " supersecondary unit".
Tertiary structure
Tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may i ...
refers to the three-dimensional structure created by a single protein molecule (a single polypeptide chain
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
). It may include one or several domains. The α-helices and β-pleated-sheets are folded into a compact globular structure
In biochemistry, globular proteins or spheroproteins are spherical ("globe-like") proteins and are one of the common protein types (the others being fibrous, disordered and membrane proteins). Globular proteins are somewhat water-soluble (form ...
. The folding is driven by the ''non-specific'' hydrophobic interactions, the burial of hydrophobic residues from water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, but the structure is stable only when the parts of a protein domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of ...
are locked into place by ''specific'' tertiary interactions, such as salt bridges, hydrogen bonds, and the tight packing of side chains and disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s. The disulfide bonds are extremely rare in cytosolic proteins, since the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
(intracellular fluid) is generally a reducing environment.
Quaternary structure
Quaternary structure is the three-dimensional structure consisting of the aggregation of two or more individual polypeptide chains (subunits) that operate as a single functional unit (multimer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relat ...
). The resulting multimer is stabilized by the same non-covalent interactions and disulfide bonds as in tertiary structure. There are many possible quaternary structure organisations. Complexes of two or more polypeptides (i.e. multiple subunits) are called multimer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relat ...
s. Specifically it would be called a dimer if it contains two subunits, a trimer if it contains three subunits, a tetramer
A tetramer () ('' tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula ...
if it contains four subunits, and a pentamer
A pentamer is an entity composed of five sub-units.
In chemistry, it applies to molecules made of five monomers.
In biochemistry, it applies to macromolecules, in particular to pentameric proteins, made of five proteic sub-units.
In microbiol ...
if it contains five subunits. The subunits are frequently related to one another by symmetry operations, such as a 2-fold axis in a dimer. Multimers made up of identical subunits are referred to with a prefix of "homo-" and those made up of different subunits are referred to with a prefix of "hetero-", for example, a heterotetramer, such as the two alpha and two beta chains of hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyt ...
.
Domains, motifs, and folds in protein structure
 Proteins are frequently described as consisting of several structural units. These units include domains, motifs, and folds. Despite the fact that there are about 100,000 different proteins expressed in
Proteins are frequently described as consisting of several structural units. These units include domains, motifs, and folds. Despite the fact that there are about 100,000 different proteins expressed in eukaryotic
Eukaryotes () are organisms whose Cell (biology), cells have a cell nucleus, nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the ...
systems, there are many fewer different domains, structural motifs and folds.
Structural domain
Astructural domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of s ...
is an element of the protein's overall structure that is self-stabilizing and often folds independently of the rest of the protein chain. Many domains are not unique to the protein products of one gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
or one gene family but instead appear in a variety of proteins. Domains often are named and singled out because they figure prominently in the biological function of the protein they belong to; for example, the "calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
-binding domain of calmodulin". Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera
Chimera, Chimaera, or Chimaira (Greek for " she-goat") originally referred to:
* Chimera (mythology), a fire-breathing monster of Ancient Lycia said to combine parts from multiple animals
* Mount Chimaera, a fire-spewing region of Lycia or Cilici ...
proteins. A conservative combination of several domains that occur in different proteins, such as protein tyrosine phosphatase
Protein tyrosine phosphatases (EC 3.1.3.48, systematic name protein-tyrosine-phosphate phosphohydrolase) are a group of enzymes that remove phosphate groups from phosphorylated tyrosine residues on proteins:
: proteintyrosine phosphate + H2O = ...
domain and C2 domain
A C2 domain is a protein structural domain involved in targeting proteins to cell membranes. The typical version (PKC-C2) has a beta-sandwich composed of 8 β-strands that co-ordinates two or three calcium ions, which bind in a cavity formed by ...
pair, was called "a superdomain" that may evolve as a single unit.
Structural and sequence motifs
Thestructural
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such ...
and sequence motifs
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''A ...
refer to short segments of protein three-dimensional structure or amino acid sequence that were found in a large number of different proteins
Supersecondary structure
Tertiary protein structures can have multiple secondary elements on the same polypeptide chain. The supersecondary structure refers to a specific combination of secondary structure elements, such as β-α-β units or ahelix-turn-helix
Helix-turn-helix is a DNA-binding protein (DBP). The helix-turn-helix (HTH) is a major structural motif capable of binding DNA. Each monomer incorporates two α helices, joined by a short strand of amino acids, that bind to the major groove of ...
motif. Some of them may be also referred to as structural motifs.
Protein fold
A protein fold refers to the general protein architecture, like a helix bundle, β-barrel,Rossmann fold
The Rossmann fold is a tertiary fold found in proteins that bind nucleotides, such as enzyme cofactors FAD, NAD+, and NADP+. This fold is composed of alternating beta strands and alpha helical segments where the beta strands are hydrogen bonde ...
or different "folds" provided in the Structural Classification of Proteins database
The Structural Classification of Proteins (SCOP) database is a largely manual classification of protein structural domains based on similarities of their structures and amino acid sequences. A motivation for this classification is to determine ...
. A related concept is protein topology
Protein topology is a property of protein molecule that does not change under deformation (without cutting or breaking a bond). Frameworks
Two main topology frameworks have been developed and applied to protein molecules. Knot Theory
Knot theory ...
.
Protein dynamics and conformational ensembles
Proteins are not static objects, but rather populate ensembles of conformational states. Transitions between these states typically occur onnanoscale
The nanoscopic scale (or nanoscale) usually refers to structures with a length scale applicable to nanotechnology, usually cited as 1–100 nanometers (nm). A nanometer is a billionth of a meter. The nanoscopic scale is (roughly speaking) a lo ...
s, and have been linked to functionally relevant phenomena such as allosteric signaling and enzyme catalysis
Enzyme catalysis is the increase in the reaction rate, rate of a process by a Biomolecule, biological molecule, an "enzyme". Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs ...
. Protein dynamics Proteins are generally thought to adopt unique structures determined by their amino acid sequences. However, proteins are not strictly static objects, but rather populate ensembles of (sometimes similar) conformations. Transitions between these stat ...
and conformational changes allow proteins to function as nanoscale biological machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for l ...
s within cells, often in the form of multi-protein complexes. Examples include motor proteins
Motor proteins are a class of molecular motors that can move along the cytoplasm of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.
Cellular function ...
, such as myosin, which is responsible for muscle contraction, kinesin
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells.
Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP) (thus kinesins are ATPases, a type of enzy ...
, which moves cargo inside cells away from the nucleus along microtubules
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 a ...
, and dynein, which moves cargo inside cells towards the nucleus and produces the axonemal beating of motile cilia
The cilium, plural cilia (), is a membrane-bound organelle found on most types of eukaryotic cell, and certain microorganisms known as ciliates. Cilia are absent in bacteria and archaea. The cilium has the shape of a slender threadlike projecti ...
and flagella. " effect, the otile ciliumis a nanomachine composed of perhaps over 600 proteins in molecular complexes, many of which also function independently as nanomachines... Flexible linkers allow the mobile protein domains connected by them to recruit their binding partners and induce long-range allostery via protein domain dynamics. "
 Proteins are often thought of as relatively stable tertiary structures that experience conformational changes after being affected by interactions with other proteins or as a part of enzymatic activity. However, proteins may have varying degrees of stability, and some of the less stable variants are
Proteins are often thought of as relatively stable tertiary structures that experience conformational changes after being affected by interactions with other proteins or as a part of enzymatic activity. However, proteins may have varying degrees of stability, and some of the less stable variants are intrinsically disordered proteins
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs ra ...
. These proteins exist and function in a relatively 'disordered' state lacking a stable tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may i ...
. As a result, they are difficult to describe by a single fixed tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may i ...
. Conformational ensembles have been devised as a way to provide a more accurate and 'dynamic' representation of the conformational state of intrinsically disordered proteins
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs ra ...
.
Protein ensemble
Ensemble may refer to:
Art
* Architectural ensemble
* Ensemble (album), ''Ensemble'' (album), Kendji Girac 2015 album
* Ensemble (band), a project of Olivier Alary
* Ensemble cast (drama, comedy)
* Ensemble (musical theatre), also known as the ...
files are a representation of a protein that can be considered to have a flexible structure. Creating these files requires determining which of the various theoretically possible protein conformations actually exist. One approach is to apply computational algorithms to the protein data in order to try to determine the most likely set of conformations for an ensemble
Ensemble may refer to:
Art
* Architectural ensemble
* Ensemble (album), ''Ensemble'' (album), Kendji Girac 2015 album
* Ensemble (band), a project of Olivier Alary
* Ensemble cast (drama, comedy)
* Ensemble (musical theatre), also known as the ...
file. There are multiple methods for preparing data for thProtein Ensemble Database
that fall into two general methodologies – pool and molecular dynamics (MD) approaches (diagrammed in the figure). The pool based approach uses the protein’s amino acid sequence to create a massive pool of random conformations. This pool is then subjected to more computational processing that creates a set of theoretical parameters for each conformation based on the structure. Conformational subsets from this pool whose average theoretical parameters closely match known experimental data for this protein are selected. The alternative molecular dynamics approach takes multiple random conformations at a time and subjects all of them to experimental data. Here the experimental data is serving as limitations to be placed on the conformations (e.g. known distances between atoms). Only conformations that manage to remain within the limits set by the experimental data are accepted. This approach often applies large amounts of experimental data to the conformations which is a very computationally demanding task. The conformational ensembles were generated for a number of highly dynamic and partially unfolded proteins, such as
Sic1
Sic1, a protein, is a stoichiometric inhibitor of Cdk1-Clb ( B-type cyclins) complexes in the budding yeast ''Saccharomyces cerevisiae''. Because B-type cyclin-Cdk1 complexes are the drivers of S-phase initiation, Sic1 prevents premature S-phase ...
/ Cdc4, p15 PAF, MKK7
Dual specificity mitogen-activated protein kinase kinase 7, also known as MAP kinase kinase 7 or MKK7, is an enzyme that in humans is encoded by the ''MAP2K7'' gene. This protein is a member of the mitogen-activated protein kinase kinase family. T ...
, Beta-synuclein and P27
Protein folding
As it is translated, polypeptides exit the ribosome mostly as arandom coil
In polymer chemistry, a random coil is a conformation of polymers where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the ch ...
and folds into its native state. The final structure of the protein chain is generally assumed to be determined by its amino acid sequence ( Anfinsen's dogma).
Protein stability
Thermodynamic stability of proteins represents the free energy difference between the folded and unfolded protein states. This free energy difference is very sensitive to temperature, hence a change in temperature may result in unfolding or denaturation.Protein denaturation
In biochemistry, denaturation is a process in which proteins or nucleic acids lose the quaternary structure, tertiary structure, and secondary structure which is present in their native state, by application of some external stress or comp ...
may result in loss of function, and loss of native state. The free energy of stabilization of soluble globular proteins typically does not exceed 50 kJ/mol. Taking into consideration the large number of hydrogen bonds that take place for the stabilization of secondary structures, and the stabilization of the inner core through hydrophobic interactions, the free energy of stabilization emerges as small difference between large numbers.
Protein structure determination

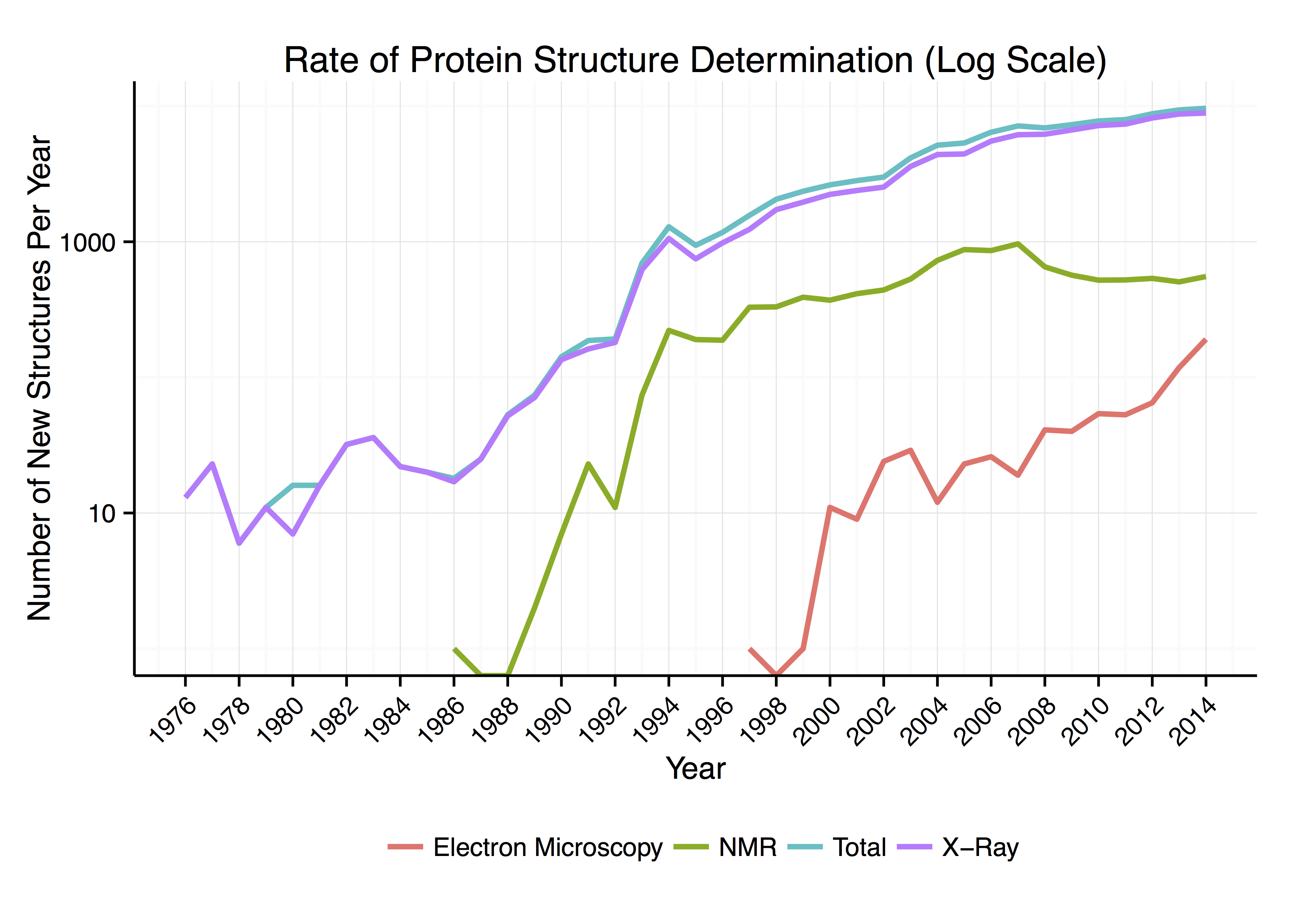
 Around 90% of the protein structures available in the
Around 90% of the protein structures available in the Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, ...
have been determined by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
. This method allows one to measure the three-dimensional (3-D) density distribution of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s in the protein, in the crystallized state, and thereby infer the 3-D coordinates of all the atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s to be determined to a certain resolution. Roughly 7% of the known protein structures have been obtained by nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
(NMR) techniques. For larger protein complexes, cryo-electron microscopy
Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample s ...
can determine protein structures. The resolution is typically lower than that of X-ray crystallography, or NMR, but the maximum resolution is steadily increasing. This technique is still a particularly valuable for very large protein complexes such as virus coat proteins and amyloid
Amyloids are aggregates of proteins characterised by a fibrillar morphology of 7–13 nm in diameter, a beta sheet (β-sheet) secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the huma ...
fibers.
General secondary structure composition can be determined via circular dichroism. Vibrational spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
can also be used to characterize the conformation of peptides, polypeptides, and proteins. Two-dimensional infrared spectroscopy has become a valuable method to investigate the structures of flexible peptides and proteins that cannot be studied with other methods. A more qualitative picture of protein structure is often obtained by proteolysis, which is also useful to screen for more crystallizable protein samples. Novel implementations of this approach, including fast parallel proteolysis (FASTpp), can probe the structured fraction and its stability without the need for purification. Once a protein's structure has been experimentally determined, further detailed studies can be done computationally, using molecular dynamic simulations of that structure.
Protein structure databases
A protein structure database is a database that is modeled around the various experimentally determined protein structures. The aim of most protein structure databases is to organize and annotate the protein structures, providing the biological community access to the experimental data in a useful way. Data included in protein structure databases often includes 3D coordinates as well as experimental information, such as unit cell dimensions and angles forx-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
determined structures. Though most instances, in this case either proteins or a specific structure determinations of a protein, also contain sequence information and some databases even provide means for performing sequence based queries, the primary attribute of a structure database is structural information, whereas sequence database
In the field of bioinformatics, a sequence database is a type of biological database that is composed of a large collection of computerized (" digital") nucleic acid sequences, protein sequences, or other polymer sequences stored on a computer. T ...
s focus on sequence information, and contain no structural information for the majority of entries. Protein structure databases are critical for many efforts in computational biology such as structure based drug design, both in developing the computational methods used and in providing a large experimental dataset used by some methods to provide insights about the function of a protein.
Structural classifications of proteins
Protein structures can be grouped based on their structural similarity, topological class or a commonevolution
Evolution is change in the heritable characteristics of biological populations over successive generations. These characteristics are the expressions of genes, which are passed on from parent to offspring during reproduction. Variation ...
ary origin. The Structural Classification of Proteins database
The Structural Classification of Proteins (SCOP) database is a largely manual classification of protein structural domains based on similarities of their structures and amino acid sequences. A motivation for this classification is to determine ...
and CATH database provide two different structural classifications of proteins. When the structural similarity is large the two proteins have possibly diverged from a common ancestor, and shared structure between proteins is considered evidence of homology. Structure similarity can then be used to group proteins together into protein superfamilies. If shared structure is significant but the fraction shared is small, the fragment shared may be the consequence of a more dramatic evolutionary event such as horizontal gene transfer
Horizontal gene transfer (HGT) or lateral gene transfer (LGT) is the movement of genetic material between unicellular and/or multicellular organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction). H ...
, and joining proteins sharing these fragments into protein superfamilies is no longer justified. Topology of a protein can be used to classify proteins as well. Knot theory and circuit topology
The circuit topology of a folded linear polymer refers to the arrangement of its intra-molecular contacts. Examples of linear polymers with intra-molecular contacts are nucleic acids and proteins. Proteins fold via formation of contacts of variou ...
are two topology frameworks developed for classification of protein folds based on chain crossing and intrachain contacts respectively.
Computational prediction of protein structure
The generation of aprotein sequence
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthes ...
is much easier than the determination of a protein structure. However, the structure of a protein gives much more insight in the function of the protein than its sequence. Therefore, a number of methods for the computational prediction of protein structure from its sequence have been developed. ''Ab initio'' prediction methods use just the sequence of the protein. Threading and homology modeling
Homology modeling, also known as comparative modeling of protein, refers to constructing an atomic-resolution model of the "''target''" protein from its amino acid sequence and an experimental three-dimensional structure of a related homologous pr ...
methods can build a 3-D model for a protein of unknown structure from experimental structures of evolutionarily-related proteins, called a protein family.
See also
*Biomolecular structure
Biomolecular structure is the intricate folded, three-dimensional shape that is formed by a molecule of protein, DNA, or RNA, and that is important to its function. The structure of these molecules may be considered at any of several length s ...
* Gene structure
Gene structure is the organisation of specialised sequence elements within a gene. Genes contain most of the information necessary for living cells to survive and reproduce. In most organisms, genes are made of DNA, where the particular DNA sequenc ...
* Nucleic acid structure
Nucleic acid structure refers to the structure of nucleic acids such as DNA and RNA. Chemically speaking, DNA and RNA are very similar. Nucleic acid structure is often divided into four different levels: primary, secondary, tertiary, and quater ...
* PCRPi-DB
Presaging Critical Residues in Protein Interfaces Database (PCRPi-DB) is a database of annotated hot spots in protein complexes for which the 3D structure is known.
See also
* Protein structure
Protein structure is the three-dimensional ar ...
* Ribbon diagram 3D schematic representation of proteins
References
Further reading
50 Years of Protein Structure Determination Timeline - HTML Version - National Institute of General Medical Sciences
at
NIH
The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late ...
External links
* {{DEFAULTSORT:Protein Structure