protein phosphatase 1 on:
[Wikipedia]
[Google]
[Amazon]
 Protein phosphatase 1 (PP1) belongs to a certain class of
Protein phosphatase 1 (PP1) belongs to a certain class of

 Protein phosphatase 1 (PP1) belongs to a certain class of
Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid monoester into a phosphate ion and an alcohol. Because a phosphatase enzyme catalyzes the hydrolysis of its substrate, it is a subcategory of hydrolases. P ...
known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
-based phosphatases. PP1 has been found to be important in the control of glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one of ...
metabolism, muscle contraction
Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such a ...
, cell progression, neuronal activities, splicing of RNA, mitosis
In cell biology, mitosis () is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is maintai ...
, cell division, apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes ( morphology) and death. These changes in ...
, protein synthesis, and regulation of membrane receptors and channels.
Structure
Each PP1 enzyme contains both acatalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
subunit and at least one regulatory
Regulation is the management of complex systems according to a set of rules and trends. In systems theory, these types of rules exist in various fields of biology and society, but the term has slightly different meanings according to context. ...
subunit. The catalytic subunit consists of a 30-kD single-domain protein that can form complexes with other regulatory subunits. The catalytic subunit is highly conserved among all eukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacter ...
, thus suggesting a common catalytic mechanism. The catalytic subunit can form complexes with various regulatory subunits. These regulatory subunits play an important role in substrate specificity as well as compartmentalization. Some common regulatory subunits include GM (PPP1R3A) and GL (PPP1R3B), which are named after their locations of action within the body (Muscle and Liver respectively). While the yeast ''S. cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been o ...
'' only encodes one catalytic subunit, mammals have four isozymes encoded by three genes, each attracting a different set of regulatory subunits.
X-ray crystallographic structural data is available for PP1 catalytic subunit. The catalytic subunit of PP1 forms an α/β fold with a central β-sandwich arranged between two α-helical domains. The interaction of the three β-sheets of the β-sandwich creates a channel for catalytic activity, as it is the site of coordination of metal ions. These metal ions have been identified as Mn and Fe and their coordination is provided by three histidines, two aspartic acids, and one asparagine.

Mechanism
The mechanism involves two metal ions binding and activating water, which initiates a nucleophilic attack on the phosphorus atom.Regulation
Regulation of these different processes is performed by distinct PP1 holoenzymes that facilitate the complexation of the PP1 catalytic subunit to various regulatory subunits. Potential inhibitors include a variety of naturally occurring toxins including okadaic acid, a diarrhetic shellfish poison, strong tumor promoter, andmicrocystin
Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria, commonly known as blue-green algae. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. C ...
. Microcystin is a liver toxin produced by blue-green algae and contains a cyclic heptapeptide structure that interacts with three distinct regions of the surface of the catalytic subunit of PP1. The structure of MCLR
Microcystin-LR (MC-LR) is a toxin produced by cyanobacteria. It is the most toxic of the microcystins.
Structure
Microcystins are cyclic heptapeptides. The seven amino acids that are involved in the structure of a microcystin include a unique � ...
does not change when complexed with PP1, but the catalytic subunit of PP1 does in order to avoid steric effects of Tyr 276 of PP1 and Mdha side chain of MCLR.
Cantharidic acid is also an inhibitor of PP1.
Biological function
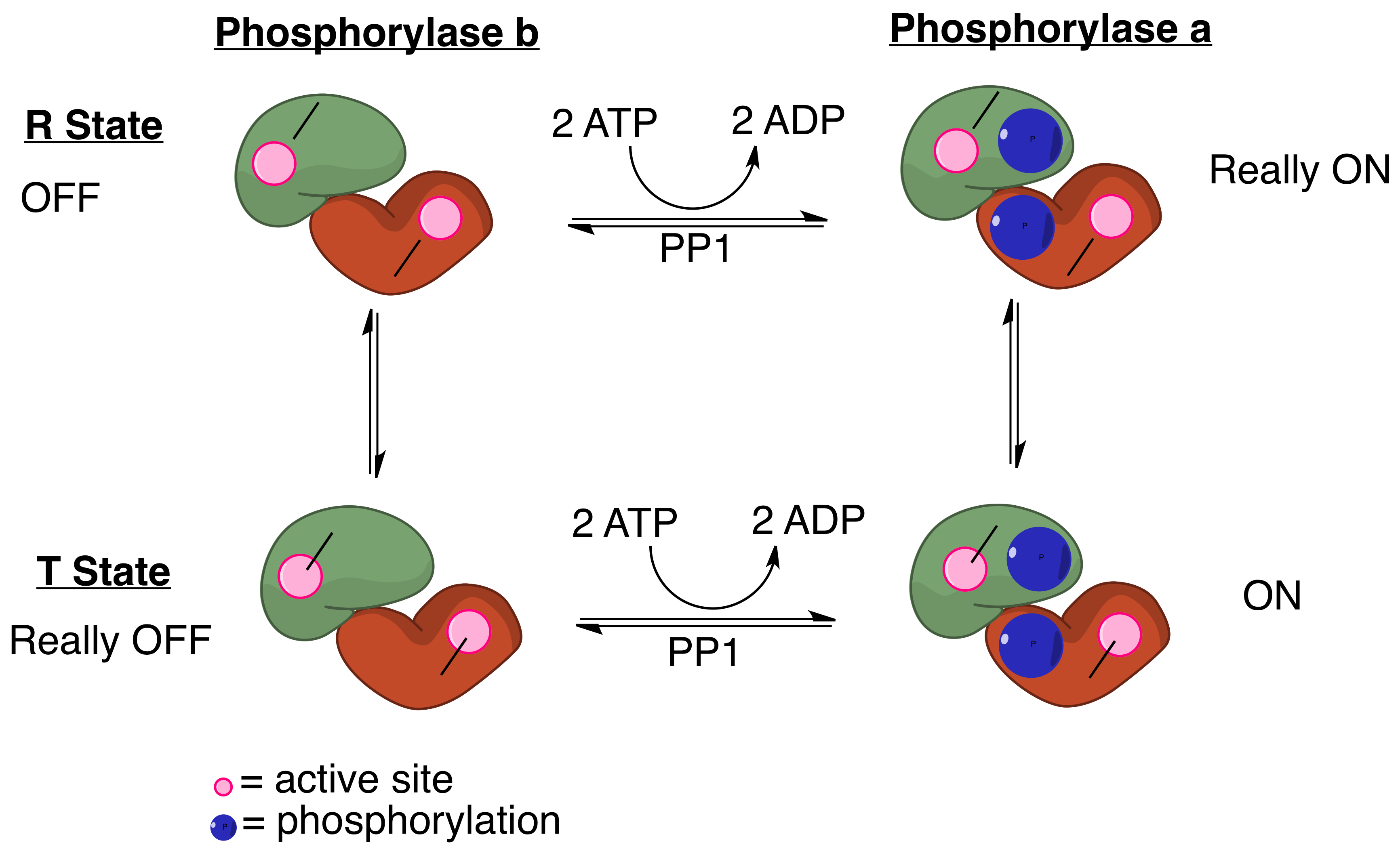
PP1 plays a crucial role in the regulation of blood-glucose levels in the liver and glycogen metabolism. PP1 is important to the reciprocal regulation of glycogen metabolism by ensuring the opposite regulation of glycogen breakdown and glycogen synthesis. Phosphorylase ''a'' serves as a glucose sensor in liver cells. When glucose levels are low, phosphorylase ''a'' in its active R state has PP1 bound tightly. This binding to phosphorylase ''a'' prevents any phosphatase activity of PP1 and maintains the glycogen phosphorylase in its active phosphorylated configuration. Therefore, there phosphorylase ''a'' will accelerate glycogen breakdown until adequate levels of glucose are achieved. When glucose concentrations get too high, phosphorylase ''a'' is converted to its inactive, T state. By shifting phosphorylase ''a'' to its T state, PP1 dissociates from the complex. This dissociation activates glycogen synthase and converts phosphorylase ''a'' to phosphorylase ''b''. Phosphorylase ''b'' does not bind PP1 allowing PP1 to remain activated. When the muscles of the body signal for the need for glycogen degradation and increased glucose concentration, PP1 will be regulated accordingly. Protein kinase A can reduce the activity of PP1. The glycogen binding region, GM, becomes phosphorylated, which causes its dissociation from the catalytic PP1 unit. This separation of the catalytic PP1 unit, glycogen, and other substrates causes a significant decrease in dephosphorylation. Also, when other substrates become phosphorylated by protein kinase A, they can bind to the catalytic subunit of PP1 and directly inhibit it. In the end, phosphorylase is kept in its active form and glycogen synthase in its inactive form.Disease relevance
In Alzheimer's, hyperphosphorylation of themicrotubule
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 1 ...
-associated protein inhibits the assembly of microtubules in neurons. Researchers at the New York State Institute for Basic Research in Developmental Disabilities showed that there is significantly lower type 1 phosphatase activity in both gray and white matters in Alzheimer disease brains. This suggests that dysfunctional phosphatases play a role in Alzheimer's disease.
Regulation of HIV-1 transcription by Protein Phosphatase 1 (PP1). It has been recognized that protein phosphatase-1 (PP1) serves as an important regulator of HIV-1 transcription. Researchers at Howard University showed that Tat protein targets PP1 to the nucleus and the consequent interaction is important for HIV-1 transcription. The protein also contributes to ebolavirus pathogenesis by dephosphorylating the viral transcription activator VP30, allowing it to produce viral mRNAs. Inhibition of PP1 prevents VP30 dephosphorylation, thus preventing manufacture of viral mRNA, and thus viral protein. The viral L polymerase is, however, still capable of replicating viral genomes without VP30 dephosphorylation by PP1.
The herpes simplex virus
Herpes simplex virus 1 and 2 (HSV-1 and HSV-2), also known by their taxonomical names '' Human alphaherpesvirus 1'' and ''Human alphaherpesvirus 2'', are two members of the human ''Herpesviridae'' family, a set of viruses that produce viral in ...
protein ICP34.5 also activates protein phosphatase 1, which overcomes the cellular stress response Cellular stress response is the wide range of molecular changes that cells undergo in response to environmental stressors, including extremes of temperature, exposure to toxins, and mechanical damage. Cellular stress responses can also be caused by ...
to viral infection; protein kinase R is activated by the virus' double-stranded RNA, and protein kinase R then phosphorylates a protein called eukaryotic initiation factor-2A (eIF-2A), which inactivates eIF-2A. EIF-2A is required for translation
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
so by shutting down eIF-2A, the cell prevents the virus from hijacking its own protein-making machinery. Herpesviruses in turn evolved ICP34.5 to defeat the defense; ICP34.5 activates protein phosphatase-1A which dephosphorylates eIF-2A, allowing translation to occur again. ICP34.5 shares the C-terminal regulatory domain () with protein phosphatase 1 subunit 15A/B.
Subunits
Protein phosphatase 1 is a multimeric enzyme that may contain the following subunits: * catalytic subunit: PPP1CA, PPP1CB, PPP1CC * regulatory subunit 1: PPP1R1A,PPP1R1B
Protein phosphatase 1 regulatory subunit 1B (PPP1R1B), also known as dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32), is a protein that in humans is encoded by the ''PPP1R1B'' gene.
Function
Midbrain dopaminergic neurons play ...
, PPP1R1C
* regulatory subunit 2: PPP1R2
* regulatory subunit 3: PPP1R3A, PPP1R3B, PPP1R3C, PPP1R3D, PPP1R3E, PPP1R3F, PPP1R3G
* regulatory subunit 7: PPP1R7
* regulatory subunit 8: PPP1R8
* regulatory subunit 9: PPP1R9A, PPP1R9B
* regulatory subunit 10: PPP1R10
* regulatory subunit 11: PPP1R11
* regulatory subunit 12: PPP1R12A, PPP1R12B
Protein phosphatase 1 regulatory subunit 12B is an enzyme that in humans is encoded by the ''PPP1R12B'' gene.
Myosin light chain phosphatase (MLCP) consists of three subunits- catalytic subunit, large subunit/myosin binding subunit (MBS) and smal ...
, PPP1R12C
* regulatory subunit 13: PPP1R13B
* regulatory subunit 14: PPP1R14A, PPP1R14B, PPP1R14C, PPP1R14D
* regulatory subunit 15: PPP1R15A, PPP1R15B
* regulatory subunit 16: PPP1R16A, PPP1R16B
As described earlier, a catalytic subunit is always paired with one or more regulatory subunits. The core sequence motif for binding to the catalytic subunit is "RVxF", but additional motifs allow for extra sites to be used. Some complexes with two regulatory subunits attached have been reported in 2002 and 2007.
References
External links
* {{Portal bar, Biology, border=no EC 3.1.3