
Protein folding is the
physical process by which a
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
chain is
translated
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
to its native
three-dimensional structure, typically a "folded"
conformation by which the protein becomes biologically functional. Via an expeditious and reproducible process, a
polypeptide folds into its characteristic three-dimensional structure from a
random coil.
Each protein exists first as an unfolded polypeptide or random coil after being translated from a sequence of
mRNA to a linear chain of
amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s. At this stage the polypeptide lacks any stable (long-lasting) three-dimensional structure (the left hand side of the first figure). As the polypeptide chain is being synthesized by a
ribosome, the linear chain begins to fold into its three-dimensional structure.
Folding of many proteins begins even during translation of the polypeptide chain. Amino acids interact with each other to produce a well-defined three-dimensional structure, the folded protein (the right hand side of the figure), known as the
native state. The resulting three-dimensional structure is determined by the amino acid sequence or primary structure (
Anfinsen's dogma
Anfinsen's dogma, also known as the thermodynamic hypothesis, is a postulate in molecular biology. It states that, at least for a small globular protein in its standard physiological environment, the native structure is determined only by the pro ...
).
The correct three-dimensional structure is essential to function, although some parts of functional proteins
may remain unfolded, so that
protein dynamics Proteins are generally thought to adopt unique structures determined by their amino acid sequences. However, proteins are not strictly static objects, but rather populate ensembles of (sometimes similar) conformations. Transitions between these stat ...
is important. Failure to fold into native structure generally produces inactive proteins, but in some instances misfolded proteins have modified or toxic functionality. Several
neurodegenerative and other
disease
A disease is a particular abnormal condition that negatively affects the structure or function of all or part of an organism, and that is not immediately due to any external injury. Diseases are often known to be medical conditions that a ...
s are believed to result from the accumulation of
amyloid fibrils formed by misfolded proteins, infectious varieties of which are known as
prions.
Many
allergies are caused by incorrect folding of some proteins, because the
immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinte ...
does not produce
antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of ...
for certain protein structures.
Denaturation of proteins is a process of transition from the folded to the
unfolded state. It happens in
cooking, in
burns, in
proteinopathies, and in other contexts.
The duration of the folding process varies dramatically depending on the protein of interest. When studied
outside the cell, the slowest folding proteins require many minutes or hours to fold primarily due to
proline isomerization, and must pass through a number of intermediate states, like checkpoints, before the process is complete. On the other hand, very small single-
domain
Domain may refer to:
Mathematics
*Domain of a function, the set of input values for which the (total) function is defined
** Domain of definition of a partial function
** Natural domain of a partial function
**Domain of holomorphy of a function
* ...
proteins with lengths of up to a hundred amino acids typically fold in a single step. Time scales of milliseconds are the norm and the very fastest known protein folding reactions are complete within a few microseconds. The folding time scale of a protein depends on its size,
contact order The contact order of a protein is a measure of the locality of the inter-amino acid contacts in the protein's native state tertiary structure. It is calculated as the average sequence distance between residues that form native contacts in the folde ...
and
circuit topology.
Understanding and simulating the protein folding process has been an important challenge for
computational biology since the late 1960s.
Process of protein folding
Primary structure
The
primary structure of a protein, its linear amino-acid sequence, determines its native conformation.
The specific amino acid residues and their position in the polypeptide chain are the determining factors for which portions of the protein fold closely together and form its three-dimensional conformation. The amino acid composition is not as important as the sequence.
The essential fact of folding, however, remains that the amino acid sequence of each protein contains the information that specifies both the native structure and the pathway to attain that state. This is not to say that nearly identical amino acid sequences always fold similarly. Conformations differ based on environmental factors as well; similar proteins fold differently based on where they are found.
Secondary structure


Formation of a
secondary structure is the first step in the folding process that a protein takes to assume its native structure. Characteristic of secondary structure are the structures known as
alpha helices and
beta sheets that fold rapidly because they are stabilized by
intramolecular hydrogen bonds, as was first characterized by
Linus Pauling. Formation of intramolecular hydrogen bonds provides another important contribution to protein stability.
α-helices are formed by hydrogen bonding of the
backbone to form a spiral shape (refer to figure on the right).
The β pleated sheet is a structure that forms with the backbone bending over itself to form the hydrogen bonds (as displayed in the figure to the left). The hydrogen bonds are between the amide hydrogen and carbonyl oxygen of the
peptide bond. There exists anti-parallel β pleated sheets and parallel β pleated sheets where the stability of the hydrogen bonds is stronger in the anti-parallel β sheet as it hydrogen bonds with the ideal 180 degree angle compared to the slanted hydrogen bonds formed by parallel sheets.
Tertiary structure
The α-Helices and β-Sheets are commonly amphipathic, meaning they have a hydrophilic and a hydrophobic portion. This ability helps in forming tertiary structure of a protein in which folding occurs so that the hydrophilic sides are facing the aqueous environment surrounding the protein and the hydrophobic sides are facing the hydrophobic core of the protein.
Secondary structure hierarchically gives way to tertiary structure formation. Once the protein's tertiary structure is formed and stabilized by the hydrophobic interactions, there may also be
covalent bonding in the form of
disulfide bridges formed between two
cysteine residues. These non-covalent and covalent contacts take a specific
topological arrangement in a native structure of a protein. Tertiary structure of a protein involves a single polypeptide chain; however, additional interactions of folded polypeptide chains give rise to quaternary structure formation.
Quaternary structure
Tertiary structure may give way to the formation of
quaternary structure in some proteins, which usually involves the "assembly" or "coassembly" of subunits that have already folded; in other words, multiple polypeptide chains could interact to form a fully functional quaternary protein.
Driving forces of protein folding

Folding is a
spontaneous process that is mainly guided by hydrophobic interactions, formation of intramolecular
hydrogen bonds,
van der Waals forces, and it is opposed by
conformational entropy
In chemical thermodynamics, conformational entropy is the entropy associated with the number of conformations of a molecule. The concept is most commonly applied to biological macromolecules such as proteins and RNA, but also be used for polysa ...
. The process of folding often begins
co-translationally, so that the
N-terminus of the protein begins to fold while the
C-terminal portion of the protein is still being
synthesized by the
ribosome; however, a protein molecule may fold spontaneously during or after
biosynthesis. While these
macromolecules may be regarded as "
folding themselves", the process also depends on the
solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
(
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
or
lipid bilayer), the concentration of
salts, the
pH, the
temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
, the possible presence of cofactors and of molecular
chaperones.
Proteins will have limitations on their folding abilities by the restricted bending angles or conformations that are possible. These allowable angles of protein folding are described with a two-dimensional plot known as the
Ramachandran plot, depicted with psi and phi angles of allowable rotation.
Hydrophobic effect
Protein folding must be thermodynamically favorable within a cell in order for it to be a spontaneous reaction. Since it is known that protein folding is a spontaneous reaction, then it must assume a negative
Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature an ...
value. Gibbs free energy in protein folding is directly related to
enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
and
entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
.
For a negative delta G to arise and for protein folding to become thermodynamically favorable, then either enthalpy, entropy, or both terms must be favorable.
Minimizing the number of hydrophobic side-chains exposed to water is an important driving force behind the folding process.
The hydrophobic effect is the phenomenon in which the hydrophobic chains of a protein collapse into the core of the protein (away from the hydrophilic environment).
In an aqueous environment, the water molecules tend to aggregate around the hydrophobic regions or side chains of the protein, creating water shells of ordered water molecules. An ordering of water molecules around a hydrophobic region increases order in a system and therefore contributes a negative change in entropy (less entropy in the system). The water molecules are fixed in these water cages which drives the
hydrophobic collapse, or the inward folding of the hydrophobic groups. The hydrophobic collapse introduces entropy back to the system via the breaking of the water cages which frees the ordered water molecules.
The multitude of hydrophobic groups interacting within the core of the globular folded protein contributes a significant amount to protein stability after folding, because of the vastly accumulated van der Waals forces (specifically
London Dispersion forces
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between a ...
).
The
hydrophobic effect exists as a driving force in thermodynamics only if there is the presence of an aqueous medium with an
amphiphilic molecule containing a large hydrophobic region. The strength of hydrogen bonds depends on their environment; thus, H-bonds enveloped in a hydrophobic core contribute more than H-bonds exposed to the aqueous environment to the stability of the native state.
In proteins with globular folds, hydrophobic amino acids tend to be interspersed along the primary sequence, rather than randomly distributed or clustered together. However, proteins that have recently been born
de novo, which tend to be
intrinsically disordered, show the opposite pattern of hydrophobic amino acid clustering along the primary sequence.
Chaperones
 Molecular chaperones
Molecular chaperones are a class of proteins that aid in the correct folding of other proteins ''
in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and p ...
''. Chaperones exist in all cellular compartments and interact with the polypeptide chain in order to allow the native three-dimensional conformation of the protein to form; however, chaperones themselves are not included in the final structure of the protein they are assisting in.
Chaperones may assist in folding even when the nascent polypeptide is being synthesized by the ribosome.
Molecular chaperones operate by binding to stabilize an otherwise unstable structure of a protein in its folding pathway, but chaperones do not contain the necessary information to know the correct native structure of the protein they are aiding; rather, chaperones work by preventing incorrect folding conformations.
In this way, chaperones do not actually increase the rate of individual steps involved in the folding pathway toward the native structure; instead, they work by reducing possible unwanted aggregations of the polypeptide chain that might otherwise slow down the search for the proper intermediate and they provide a more efficient pathway for the polypeptide chain to assume the correct conformations.
Chaperones are not to be confused with folding
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
proteins, which catalyze chemical reactions responsible for slow steps in folding pathways. Examples of folding catalysts are protein
disulfide isomerases and
peptidyl-prolyl isomerases that may be involved in formation of
disulfide bonds or interconversion between cis and trans stereoisomers of peptide group.
Chaperones are shown to be critical in the process of protein folding ''in vivo'' because they provide the protein with the aid needed to assume its proper alignments and conformations efficiently enough to become "biologically relevant".
This means that the polypeptide chain could theoretically fold into its native structure without the aid of chaperones, as demonstrated by protein folding experiments conducted ''
in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called " test-tube experiments", these studies in biology a ...
'';
however, this process proves to be too inefficient or too slow to exist in biological systems; therefore, chaperones are necessary for protein folding ''in vivo.'' Along with its role in aiding native structure formation, chaperones are shown to be involved in various roles such as protein transport, degradation, and even allow
denatured proteins exposed to certain external denaturant factors an opportunity to refold into their correct native structures.
A fully denatured protein lacks both tertiary and secondary structure, and exists as a so-called
random coil. Under certain conditions some proteins can refold; however, in many cases, denaturation is irreversible.
Cells sometimes protect their proteins against the denaturing influence of heat with
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s known as
heat shock protein
Heat shock proteins (HSP) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including expo ...
s (a type of chaperone), which assist other proteins both in folding and in remaining folded.
Heat shock protein
Heat shock proteins (HSP) are a family of proteins produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including expo ...
s have been found in all species examined, from
bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
to humans, suggesting that they evolved very early and have an important function. Some proteins never fold in cells at all except with the assistance of chaperones which either isolate individual proteins so that their folding is not interrupted by interactions with other proteins or help to unfold misfolded proteins, allowing them to refold into the correct native structure.
This function is crucial to prevent the risk of
precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. ...
into
insoluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
amorphous aggregates. The external factors involved in protein denaturation or disruption of the native state include temperature, external fields (electric, magnetic),
molecular crowding,
and even the limitation of space (i.e. confinement), which can have a big influence on the folding of proteins. High concentrations of
solutes
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solven ...
, extremes of
pH, mechanical forces, and the presence of chemical denaturants can contribute to protein denaturation, as well. These individual factors are categorized together as stresses. Chaperones are shown to exist in increasing concentrations during times of cellular stress and help the proper folding of emerging proteins as well as denatured or misfolded ones.
Under some conditions proteins will not fold into their biochemically functional forms. Temperatures above or below the range that cells tend to live in will cause
thermally unstable proteins to unfold or denature (this is why boiling makes an
egg white turn opaque). Protein thermal stability is far from constant, however; for example,
hyperthermophilic bacteria have been found that grow at temperatures as high as 122 °C, which of course requires that their full complement of vital proteins and protein assemblies be stable at that temperature or above.
The bacterium ''
E. coli'' is the host for
bacteriophage T4, and the phage encoded gp31 protein () appears to be structurally and functionally homologous to ''E. coli''
chaperone protein
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to as ...
GroES and able to substitute for it in the assembly of bacteriophage T4
virus
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsk ...
particles during infection.
[Marusich EI, Kurochkina LP, Mesyanzhinov VV. Chaperones in bacteriophage T4 assembly. Biochemistry (Mosc). 1998;63(4):399-406] Like GroES, gp31 forms a stable complex with
GroEL chaperonin that is absolutely necessary for the folding and assembly in vivo of the bacteriophage T4 major capsid protein gp23.
[
]
Fold switching
Some proteins have multiple native structures, and change their fold based on some external factors. For example, the KaiB protein switches fold throughout the day, acting as a clock for cyanobacteria. It has been estimated that around 0.5–4% of PDB (Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, cr ...
) proteins switch folds.
Protein misfolding and neurodegenerative disease
A protein is considered to be misfolded
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
if it cannot achieve its normal native state. This can be due to mutations in the amino acid sequence or a disruption of the normal folding process by external factors.β-sheets
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a gen ...
that are organized in a supramolecular arrangement known as a cross-β structure. These β-sheet-rich assemblies are very stable, very insoluble, and generally resistant to proteolysis.Parkinson's disease
Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms beco ...
.fibril
Fibrils (from the Latin ''fibra'') are structural biological materials found in nearly all living organisms. Not to be confused with fibers or filaments, fibrils tend to have diameters ranging from 10-100 nanometers (whereas fibers are micro ...
s. It is not completely clear whether the aggregates are the cause or merely a reflection of the loss of protein homeostasis, the balance between synthesis, folding, aggregation and protein turnover. Recently the European Medicines Agency approved the use of Tafamidis or Vyndaqel (a kinetic stabilizer of tetrameric transthyretin) for the treatment of transthyretin amyloid diseases. This suggests that the process of amyloid fibril formation (and not the fibrils themselves) causes the degeneration of post-mitotic tissue in human amyloid diseases.antitrypsin
Alpha-1 antitrypsin or α1-antitrypsin (A1AT, α1AT, A1A, or AAT) is a protein belonging to the serpin superfamily. It is encoded in humans by the ''SERPINA1'' gene. A protease inhibitor, it is also known as alpha1–proteinase inhibitor (A1PI) ...
-associated emphysema, cystic fibrosis and the lysosomal storage diseases
Lysosomal storage diseases (LSDs; ) are a group of over 70 rare inherited metabolic disorders that result from defects in lysosomal function. Lysosomes are sacs of enzymes within cells that digest large molecules and pass the fragments on to other ...
, where loss of function is the origin of the disorder. While protein replacement therapy has historically been used to correct the latter disorders, an emerging approach is to use pharmaceutical chaperones to fold mutated proteins to render them functional.
Experimental techniques for studying protein folding
While inferences about protein folding can be made through mutation studies, typically, experimental techniques for studying protein folding rely on the gradual unfolding or folding of proteins and observing conformational changes using standard non-crystallographic techniques.
X-ray crystallography
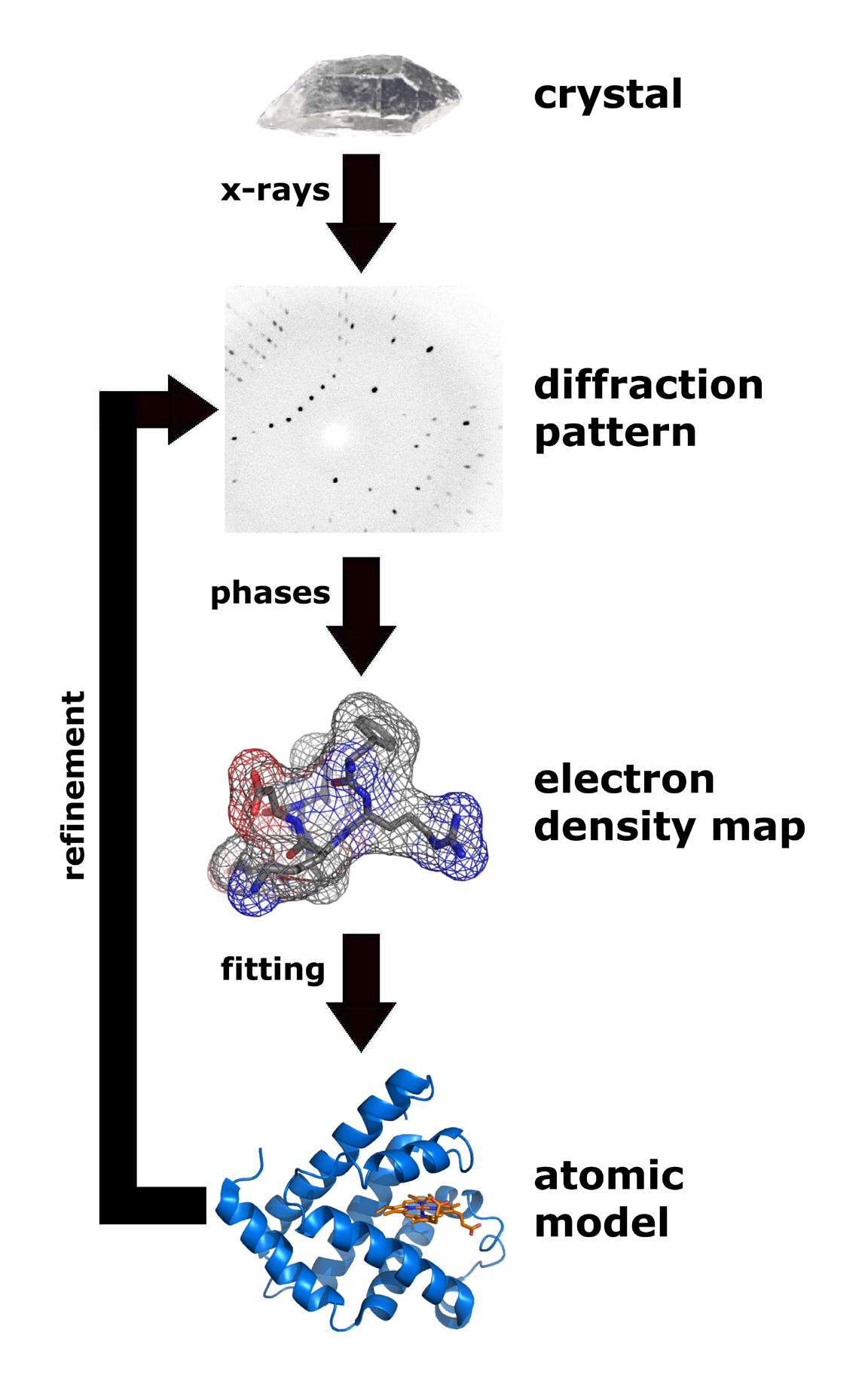 X-ray crystallography is one of the more efficient and important methods for attempting to decipher the three dimensional configuration of a folded protein.
X-ray crystallography is one of the more efficient and important methods for attempting to decipher the three dimensional configuration of a folded protein.
Fluorescence spectroscopy
Fluorescence spectroscopy is a highly sensitive method for studying the folding state of proteins. Three amino acids, phenylalanine (Phe), tyrosine (Tyr) and tryptophan (Trp), have intrinsic fluorescence properties, but only Tyr and Trp are used experimentally because their quantum yields are high enough to give good fluorescence signals. Both Trp and Tyr are excited by a wavelength of 280 nm, whereas only Trp is excited by a wavelength of 295 nm. Because of their aromatic character, Trp and Tyr residues are often found fully or partially buried in the hydrophobic core of proteins, at the interface between two protein domains, or at the interface between subunits of oligomeric proteins. In this apolar environment, they have high quantum yields and therefore high fluorescence intensities. Upon disruption of the protein's tertiary or quaternary structure, these side chains become more exposed to the hydrophilic environment of the solvent, and their quantum yields decrease, leading to low fluorescence intensities. For Trp residues, the wavelength of their maximal fluorescence emission also depend on their environment.
Fluorescence spectroscopy can be used to characterize the equilibrium unfolding
In biochemistry, equilibrium unfolding is the process of unfolding a protein or RNA molecule by gradually changing its environment, such as by changing the temperature or pressure, pH, adding chemical denaturants, or applying force as with an ...
of proteins by measuring the variation in the intensity of fluorescence emission or in the wavelength of maximal emission as functions of a denaturant value.
Circular dichroism
Circular dichroism is one of the most general and basic tools to study protein folding. Circular dichroism spectroscopy measures the absorption of circularly polarized light
In electrodynamics, circular polarization of an electromagnetic wave is a polarization state in which, at each point, the electromagnetic field of the wave has a constant magnitude and is rotating at a constant rate in a plane perpendicular to t ...
. In proteins, structures such as alpha helices and beta sheets are chiral, and thus absorb such light. The absorption of this light acts as a marker of the degree of foldedness of the protein ensemble. This technique has been used to measure equilibrium unfolding
In biochemistry, equilibrium unfolding is the process of unfolding a protein or RNA molecule by gradually changing its environment, such as by changing the temperature or pressure, pH, adding chemical denaturants, or applying force as with an ...
of the protein by measuring the change in this absorption as a function of denaturant concentration or temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
. A denaturant melt measures the free energy of unfolding as well as the protein's m value, or denaturant dependence. A temperature melt measures the denaturation temperature (Tm) of the protein.kinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical kin ...
and to generate chevron plots.
Vibrational circular dichroism of proteins
The more recent developments of vibrational circular dichroism Vibrational circular dichroism (VCD) is a spectroscopic technique which detects differences in attenuation of left and right circularly polarized light passing through a sample. It is the extension of circular dichroism spectroscopy into the infrar ...
(VCD) techniques for proteins, currently involving Fourier transform (FT) instruments, provide powerful means for determining protein conformations in solution even for very large protein molecules. Such VCD studies of proteins can be combined with X-ray diffraction data for protein crystals, FT-IR data for protein solutions in heavy water (D2O), or quantum computations.
Protein nuclear magnetic resonance spectroscopy
Protein nuclear magnetic resonance (NMR) is able to collect protein structural data by inducing a magnet field through samples of concentrated protein. In NMR, depending on the chemical environment, certain nuclei will absorb specific radio-frequencies.HSQC The heteronuclear single quantum coherence or heteronuclear single quantum correlation experiment, normally abbreviated as HSQC, is used frequently in NMR spectroscopy of organic molecules and is of particular significance in the field of protein NM ...
, time relaxation (T1 & T2), and NOE. Because protein folding takes place in about 50 to 3000 s−1 CPMG Relaxation dispersion and chemical exchange saturation transfer have become some of the primary techniques for NMR analysis of folding.
Because protein folding takes place in about 50 to 3000 s−1 CPMG Relaxation dispersion and chemical exchange saturation transfer have become some of the primary techniques for NMR analysis of folding.SOD1
Superoxide dismutase u-Zn'' also known as superoxide dismutase 1 or hSod1 is an enzyme that in humans is encoded by the ''SOD1'' gene, located on chromosome 21. SOD1 is one of three human superoxide dismutases. It is implicated in apoptosis, fa ...
, excited intermediates were studied with relaxation dispersion and Saturation transfer.
Dual-polarization interferometry
Dual polarisation interferometry is a surface-based technique for measuring the optical properties of molecular layers. When used to characterize protein folding, it measures the conformation by determining the overall size of a monolayer of the protein and its density in real time at sub-Angstrom resolution,temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
.
Studies of folding with high time resolution
The study of protein folding has been greatly advanced in recent years by the development of fast, time-resolved techniques. Experimenters rapidly trigger the folding of a sample of unfolded protein and observe the resulting dynamics. Fast techniques in use include neutron scattering,Sheena Radford
Sheena Elizabeth Radford FRS FMedSci is a British biophysicist, and Astbury Professor of Biophysics in the Astbury Centre for Structural Molecular Biology, School of Molecular and Cellular Biology at the University of Leeds. Radford is the A ...
, Chris Dobson
Sir Christopher Martin Dobson (8 October 1949 – 8 September 2019) was a British chemist, who was the John Humphrey Plummer Professor of Chemical and Structural Biology in the Department of Chemistry at the University of Cambridge, and Mast ...
, Alan Fersht, Bengt Nölting and Lars Konermann.
Proteolysis
Proteolysis is routinely used to probe the fraction unfolded under a wide range of solution conditions (e.g. fast parallel proteolysis (FASTpp)
Fast parallel proteolysis (FASTpp) is a method to determine the thermostability of proteins by measuring which fraction of protein resists rapid proteolytic digestion.
History and background
Proteolysis is widely used in biochemistry and cell ...
.
Single-molecule force spectroscopy
Single molecule techniques such as optical tweezers and AFM have been used to understand protein folding mechanisms of isolated proteins as well as proteins with chaperones.
Biotin painting
Biotin painting enables condition-specific cellular snapshots of (un)folded proteins. Biotin 'painting' shows a bias towards predicted Intrinsically disordered proteins.
Computational studies of protein folding
Computational studies of protein folding includes three main aspects related to the prediction of protein stability, kinetics, and structure. A 2013 review summarizes the available computational methods for protein folding.
Levinthal's paradox
In 1969, Cyrus Levinthal noted that, because of the very large number of degrees of freedom in an unfolded polypeptide chain, the molecule has an astronomical number of possible conformations. An estimate of 3300 or 10143 was made in one of his papers. Levinthal's paradox is a thought experiment based on the observation that if a protein were folded by sequential sampling of all possible conformations, it would take an astronomical amount of time to do so, even if the conformations were sampled at a rapid rate (on the nanosecond or picosecond scale). Based upon the observation that proteins fold much faster than this, Levinthal then proposed that a random conformational search does not occur, and the protein must, therefore, fold through a series of meta-stable intermediate states.
Energy landscape of protein folding
 The configuration space of a protein during folding can be visualized as an
The configuration space of a protein during folding can be visualized as an energy landscape
An energy landscape is a mapping of possible states of a system. The concept is frequently used in physics, chemistry, and biochemistry, e.g. to describe all possible conformations of a molecular entity, or the spatial positions of interacting ...
. According to Joseph Bryngelson and Peter Wolynes, proteins follow the ''principle of minimal frustration'', meaning that naturally evolved proteins have optimized their folding energy landscapes,folding funnel
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells. ...
" landscape allows the protein to fold to the native state through any of a large number of pathways and intermediates, rather than being restricted to a single mechanism. The theory is supported by both computational simulations of model proteins and experimental studies,design
A design is a plan or specification for the construction of an object or system or for the implementation of an activity or process or the result of that plan or specification in the form of a prototype, product, or process. The verb ''to design' ...
.
Modeling of protein folding
'' De novo'' or '' ab initio'' techniques for computational protein structure prediction can be used for simulating various aspects of protein folding. Molecular Dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of th ...
(MD) was used in simulations of protein folding and dynamics in silico.
/ref> target protein folding.
Long continuous-trajectory simulations have been performed on Anton, a massively parallel supercomputer designed and built around custom ASICs and interconnects by D. E. Shaw Research
D. E. Shaw Research (DESRES) is a privately held biochemistry research company based in New York City. Under the scientific direction of David E. Shaw, the group's chief scientist, D. E. Shaw Research develops technologies for molecular dyn ...
. The longest published result of a simulation performed using Anton is a 2.936 millisecond simulation of NTL9 at 355 K.
See also
* Chevron plot
* Denaturation midpoint
Denaturation midpoint of a protein is defined as the temperature (Tm) or concentration of denaturant (Cm) at which both the folded and unfolded states are equally populated at equilibrium (assuming two-state protein folding). Tm is often deter ...
* Downhill folding
Downhill folding is a process in which a protein folds without encountering any significant macroscopic free energy barrier. It is a key prediction of the folding funnel hypothesis of the energy landscape theory of proteins.
Overview
Downhill ...
* Folding (chemistry)
* Phi value analysis
* Potential energy of protein
* Protein dynamics Proteins are generally thought to adopt unique structures determined by their amino acid sequences. However, proteins are not strictly static objects, but rather populate ensembles of (sometimes similar) conformations. Transitions between these stat ...
* Protein misfolding cyclic amplification
* Protein structure prediction software
This list of protein structure prediction software summarizes notable used software tools in protein structure prediction, including homology modeling, protein threading, ''ab initio'' methods, secondary structure prediction, and transmembrane h ...
* Proteopathy
* Time-resolved mass spectrometry
References
External links
Human Proteome Folding Project
{{Authority control
Protein structure
 Protein folding is the physical process by which a
Protein folding is the physical process by which a 
 Formation of a secondary structure is the first step in the folding process that a protein takes to assume its native structure. Characteristic of secondary structure are the structures known as alpha helices and beta sheets that fold rapidly because they are stabilized by intramolecular hydrogen bonds, as was first characterized by Linus Pauling. Formation of intramolecular hydrogen bonds provides another important contribution to protein stability. α-helices are formed by hydrogen bonding of the backbone to form a spiral shape (refer to figure on the right). The β pleated sheet is a structure that forms with the backbone bending over itself to form the hydrogen bonds (as displayed in the figure to the left). The hydrogen bonds are between the amide hydrogen and carbonyl oxygen of the peptide bond. There exists anti-parallel β pleated sheets and parallel β pleated sheets where the stability of the hydrogen bonds is stronger in the anti-parallel β sheet as it hydrogen bonds with the ideal 180 degree angle compared to the slanted hydrogen bonds formed by parallel sheets.
Formation of a secondary structure is the first step in the folding process that a protein takes to assume its native structure. Characteristic of secondary structure are the structures known as alpha helices and beta sheets that fold rapidly because they are stabilized by intramolecular hydrogen bonds, as was first characterized by Linus Pauling. Formation of intramolecular hydrogen bonds provides another important contribution to protein stability. α-helices are formed by hydrogen bonding of the backbone to form a spiral shape (refer to figure on the right). The β pleated sheet is a structure that forms with the backbone bending over itself to form the hydrogen bonds (as displayed in the figure to the left). The hydrogen bonds are between the amide hydrogen and carbonyl oxygen of the peptide bond. There exists anti-parallel β pleated sheets and parallel β pleated sheets where the stability of the hydrogen bonds is stronger in the anti-parallel β sheet as it hydrogen bonds with the ideal 180 degree angle compared to the slanted hydrogen bonds formed by parallel sheets.
 Folding is a spontaneous process that is mainly guided by hydrophobic interactions, formation of intramolecular hydrogen bonds, van der Waals forces, and it is opposed by
Folding is a spontaneous process that is mainly guided by hydrophobic interactions, formation of intramolecular hydrogen bonds, van der Waals forces, and it is opposed by  Molecular chaperones are a class of proteins that aid in the correct folding of other proteins ''
Molecular chaperones are a class of proteins that aid in the correct folding of other proteins '' X-ray crystallography is one of the more efficient and important methods for attempting to decipher the three dimensional configuration of a folded protein. To be able to conduct X-ray crystallography, the protein under investigation must be located inside a crystal lattice. To place a protein inside a crystal lattice, one must have a suitable solvent for crystallization, obtain a pure protein at supersaturated levels in solution, and precipitate the crystals in solution. Once a protein is crystallized, X-ray beams can be concentrated through the crystal lattice which would diffract the beams or shoot them outwards in various directions. These exiting beams are correlated to the specific three-dimensional configuration of the protein enclosed within. The X-rays specifically interact with the electron clouds surrounding the individual atoms within the protein crystal lattice and produce a discernible diffraction pattern. Only by relating the electron density clouds with the amplitude of the X-rays can this pattern be read and lead to assumptions of the phases or phase angles involved that complicate this method. Without the relation established through a mathematical basis known as Fourier transform, the " phase problem" would render predicting the diffraction patterns very difficult. Emerging methods like multiple isomorphous replacement use the presence of a heavy metal ion to diffract the X-rays into a more predictable manner, reducing the number of variables involved and resolving the phase problem.
X-ray crystallography is one of the more efficient and important methods for attempting to decipher the three dimensional configuration of a folded protein. To be able to conduct X-ray crystallography, the protein under investigation must be located inside a crystal lattice. To place a protein inside a crystal lattice, one must have a suitable solvent for crystallization, obtain a pure protein at supersaturated levels in solution, and precipitate the crystals in solution. Once a protein is crystallized, X-ray beams can be concentrated through the crystal lattice which would diffract the beams or shoot them outwards in various directions. These exiting beams are correlated to the specific three-dimensional configuration of the protein enclosed within. The X-rays specifically interact with the electron clouds surrounding the individual atoms within the protein crystal lattice and produce a discernible diffraction pattern. Only by relating the electron density clouds with the amplitude of the X-rays can this pattern be read and lead to assumptions of the phases or phase angles involved that complicate this method. Without the relation established through a mathematical basis known as Fourier transform, the " phase problem" would render predicting the diffraction patterns very difficult. Emerging methods like multiple isomorphous replacement use the presence of a heavy metal ion to diffract the X-rays into a more predictable manner, reducing the number of variables involved and resolving the phase problem.
 Because protein folding takes place in about 50 to 3000 s−1 CPMG Relaxation dispersion and chemical exchange saturation transfer have become some of the primary techniques for NMR analysis of folding. In addition, both techniques are used to uncover excited intermediate states in the protein folding landscape. To do this, CPMG Relaxation dispersion takes advantage of the spin echo phenomenon. This technique exposes the target nuclei to a 90 pulse followed by one or more 180 pulses. As the nuclei refocus, a broad distribution indicates the target nuclei is involved in an intermediate excited state. By looking at Relaxation dispersion plots the data collect information on the thermodynamics and kinetics between the excited and ground. Saturation Transfer measures changes in signal from the ground state as excited states become perturbed. It uses weak radio frequency irradiation to saturate the excited state of a particular nuclei which transfers its saturation to the ground state. This signal is amplified by decreasing the magnetization (and the signal) of the ground state.
The main limitations in NMR is that its resolution decreases with proteins that are larger than 25 kDa and is not as detailed as X-ray crystallography. Additionally, protein NMR analysis is quite difficult and can propose multiple solutions from the same NMR spectrum.
In a study focused on the folding of an amyotrophic lateral sclerosis involved protein
Because protein folding takes place in about 50 to 3000 s−1 CPMG Relaxation dispersion and chemical exchange saturation transfer have become some of the primary techniques for NMR analysis of folding. In addition, both techniques are used to uncover excited intermediate states in the protein folding landscape. To do this, CPMG Relaxation dispersion takes advantage of the spin echo phenomenon. This technique exposes the target nuclei to a 90 pulse followed by one or more 180 pulses. As the nuclei refocus, a broad distribution indicates the target nuclei is involved in an intermediate excited state. By looking at Relaxation dispersion plots the data collect information on the thermodynamics and kinetics between the excited and ground. Saturation Transfer measures changes in signal from the ground state as excited states become perturbed. It uses weak radio frequency irradiation to saturate the excited state of a particular nuclei which transfers its saturation to the ground state. This signal is amplified by decreasing the magnetization (and the signal) of the ground state.
The main limitations in NMR is that its resolution decreases with proteins that are larger than 25 kDa and is not as detailed as X-ray crystallography. Additionally, protein NMR analysis is quite difficult and can propose multiple solutions from the same NMR spectrum.
In a study focused on the folding of an amyotrophic lateral sclerosis involved protein