Potential Energy Surface on:
[Wikipedia]
[Google]
[Amazon]
A potential energy surface (PES) describes the 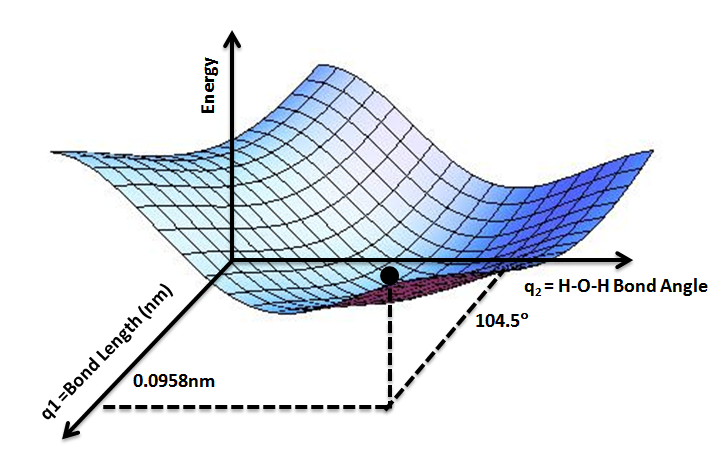 The PES concept finds application in fields such as
The PES concept finds application in fields such as
Errol G. Lewars, 2nd ed. (Springer 2011) p.21 The first semi-empirical calculation of a potential energy surface was proposed for the H + H2 reaction by Henry Eyring and Michael Polanyi in 1931. Eyring used potential energy surfaces to calculate
 We have different relevant elements in the 2-D PES:
* The 2-D plot shows the minima points where we find reactants, the products and the saddle point or transition state.
* The transition state is a maximum in the reaction coordinate and a minimum in the coordinate perpendicular to the reaction path.
* The advance of time describes a trajectory in every reaction. Depending on the conditions of the reaction the process will show different ways to get to the product formation plotted between the 2 axes.
We have different relevant elements in the 2-D PES:
* The 2-D plot shows the minima points where we find reactants, the products and the saddle point or transition state.
* The transition state is a maximum in the reaction coordinate and a minimum in the coordinate perpendicular to the reaction path.
* The advance of time describes a trajectory in every reaction. Depending on the conditions of the reaction the process will show different ways to get to the product formation plotted between the 2 axes.
energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of ...
of a system
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its environment, is described by its boundaries, structure and purpose and express ...
, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential.
It is helpful to use the analogy of a landscape: for a system with two degrees of freedom
Degrees of freedom (often abbreviated df or DOF) refers to the number of independent variables or parameters of a thermodynamic system. In various scientific fields, the word "freedom" is used to describe the limits to which physical movement or ...
(e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground).
 The PES concept finds application in fields such as
The PES concept finds application in fields such as chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
and physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which ...
, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
or computing the rates
Rate or rates may refer to:
Finance
* Rates (tax), a type of taxation system in the United Kingdom used to fund local government
* Exchange rate, rate at which one currency will be exchanged for another
Mathematics and science
* Rate (mathema ...
of a chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
.
Mathematical definition and computation
The geometry of a set of atoms can be described by a vector, , whose elements represent the atom positions. The vector could be the set of theCartesian coordinates
A Cartesian coordinate system (, ) in a plane is a coordinate system that specifies each point uniquely by a pair of numerical coordinates, which are the signed distances to the point from two fixed perpendicular oriented lines, measured in ...
of the atoms, or could also be a set of inter-atomic distances and angles.
Given , the energy as a function of the positions, , is the value of for all of interest. Using the landscape analogy from the introduction, ''E'' gives the height on the "energy landscape" so that the concept of a potential energy ''surface'' arises.
To study a chemical reaction using the PES as a function of atomic positions, it is necessary to calculate the energy for every atomic arrangement of interest. Methods of calculating the energy of a particular atomic arrangement of atoms are well described in the computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of mo ...
article, and the emphasis here will be on finding approximations of to yield fine-grained energy-position information.
For very simple chemical systems or when simplifying approximations are made about inter-atomic interactions, it is sometimes possible to use an analytically derived expression for the energy as a function of the atomic positions. An example is the London
London is the capital and List of urban areas in the United Kingdom, largest city of England and the United Kingdom, with a population of just under 9 million. It stands on the River Thames in south-east England at the head of a estuary dow ...
- Eyring- Polanyi-Sato potential for the system H + H2 as a function of the three H-H distances.
For more complicated systems, calculation of the energy of a particular arrangement of atoms is often too computationally expensive for large scale representations of the surface to be feasible. For these systems a possible approach is to calculate only a reduced set of points on the PES and then use a computationally cheaper interpolation method, for example Shepard interpolation, to fill in the gaps.
Application
A PES is a conceptual tool for aiding the analysis ofmolecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that dete ...
and chemical reaction dynamics. Once the necessary points are evaluated on a PES, the points can be classified according to the first and second derivatives of the energy with respect to position, which respectively are the gradient
In vector calculus, the gradient of a scalar-valued differentiable function of several variables is the vector field (or vector-valued function) \nabla f whose value at a point p is the "direction and rate of fastest increase". If the gr ...
and the curvature
In mathematics, curvature is any of several strongly related concepts in geometry. Intuitively, the curvature is the amount by which a curve deviates from being a straight line, or a surface deviates from being a plane.
For curves, the can ...
. Stationary points (or points with a zero gradient) have physical meaning: energy minima correspond to physically stable chemical species and saddle point
In mathematics, a saddle point or minimax point is a point on the surface of the graph of a function where the slopes (derivatives) in orthogonal directions are all zero (a critical point), but which is not a local extremum of the functi ...
s correspond to transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
s, the highest energy point on the reaction coordinate (which is the lowest energy pathway connecting a chemical reactant to a chemical product).
Attractive and repulsive surfaces
Potential energy surfaces for chemical reactions can be classified as ''attractive'' or ''repulsive'' by comparing the extensions of the bond lengths in the activated complex relative to those of the reactants and products. For a reaction of type A + B—C → A—B + C, the bond length extension for the newly formed A—B bond is defined as R*AB = RAB − R0AB, where RAB is the A—B bond length in the transition state and R0AB in the product molecule. Similarly for the bond which is broken in the reaction, R*BC = RBC − R0BC, where R0BC refers to the reactant molecule.Keith J. Laidler
Keith James Laidler (January 3, 1916 – August 26, 2003), born in England, was notable as a pioneer in chemical kinetics and authority on the physical chemistry of enzymes.
Education
Laidler received his early education at Liverpool College. H ...
, ''Chemical Kinetics'' (3rd ed., Harper & Row 1987) p.461-8
For exothermic reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines ...
s, a PES is classified as ''attractive'' (or ''early-downhill'') if R*AB > R*BC, so that the transition state is reached while the reactants are approaching each other. After the transition state, the A—B bond length continues to decrease, so that much of the liberated reaction energy is converted into vibrational energy of the A—B bond.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 1998) p.272-4 An example is the harpoon reaction K + Br2 → K—Br + Br, in which the initial long-range attraction of the reactants leads to an activated complex resembling K+•••Br−•••Br. The vibrationally excited populations of product molecules can be detected by infrared chemiluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊,
: + -> lozenge - ...
.
In contrast the PES for the reaction H + Cl2 → HCl + Cl is ''repulsive'' (or ''late-downhill'') because R*HCl < R*ClCl and the transition state is reached when the products are separating. For this reaction in which the atom A (here H) is lighter than B and C, the reaction energy is released primarily as translational kinetic energy
In physics, the kinetic energy of an object is the energy that it possesses due to its motion.
It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acce ...
of the products. For a reaction such as F + H2 → HF + H in which atom A is heavier than B and C, there is ''mixed'' energy release, both vibrational and translational, even though the PES is repulsive.
For endothermic reactions, the type of surface determines the type of energy which is most effective in bringing about reaction. Translational energy of the reactants is most effective at inducing reactions with an attractive surface, while vibrational excitation (to higher vibrational quantum number v) is more effective for reactions with a repulsive surface. As an example of the latter case, the reaction F + HCl(v=1) → Cl + HF is about five times faster than F + HCl(v=0) → Cl + HF for the same total energy of HCl.Atkins P. and de Paula J. ''Physical Chemistry'' (8th ed., W.H.Freeman 2006) p.889-890
History
The concept of a potential energy surface for chemical reactions was first suggested by the French physicist René Marcelin in 1913.Computational Chemistry: Introduction to the Theory and Applications of Molecular and Quantum MechanicsErrol G. Lewars, 2nd ed. (Springer 2011) p.21 The first semi-empirical calculation of a potential energy surface was proposed for the H + H2 reaction by Henry Eyring and Michael Polanyi in 1931. Eyring used potential energy surfaces to calculate
reaction rate constant In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction.
For a reaction between reactants A and B to form product C
the reaction rate is often found to have the ...
s in the transition state theory in 1935.
H + H2 two-dimensional PES
Potential energy surfaces are commonly shown as three-dimensional graphs, but they can also be represented by two-dimensional graphs, in which the advancement of the reaction is plotted by the use of isoenergetic lines. The collinear system H + H2 is a simple reaction that allows a two-dimension PES to be plotted in an easy and understandable way. In this reaction, a hydrogen atom (H) reacts with a dihydrogen molecule (H2) by forming a new bond with one atom from the molecule, which in turn breaks the bond of the original molecule. This is symbolized as Ha + Hb–Hc → Ha–Hb + Hc. The progression of the reaction from reactants (H+H₂) to products (H-H-H), as well as the energy of the species that take part in the reaction, are well defined in the corresponding potential energy surface. Energy profiles describe potential energy as a function of geometrical variables (PES in any dimension are independent of time and temperature). We have different relevant elements in the 2-D PES:
* The 2-D plot shows the minima points where we find reactants, the products and the saddle point or transition state.
* The transition state is a maximum in the reaction coordinate and a minimum in the coordinate perpendicular to the reaction path.
* The advance of time describes a trajectory in every reaction. Depending on the conditions of the reaction the process will show different ways to get to the product formation plotted between the 2 axes.
We have different relevant elements in the 2-D PES:
* The 2-D plot shows the minima points where we find reactants, the products and the saddle point or transition state.
* The transition state is a maximum in the reaction coordinate and a minimum in the coordinate perpendicular to the reaction path.
* The advance of time describes a trajectory in every reaction. Depending on the conditions of the reaction the process will show different ways to get to the product formation plotted between the 2 axes.
See also
*Computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of mo ...
* Energy minimization (or geometry optimization)
* Energy profile (chemistry)
* Reaction coordinate
References
{{Reaction mechanisms Quantum mechanics Potential theory Quantum chemistry