potassium gold cyanide on:
[Wikipedia]
[Google]
[Amazon]
Potassium dicyanoaurate is an
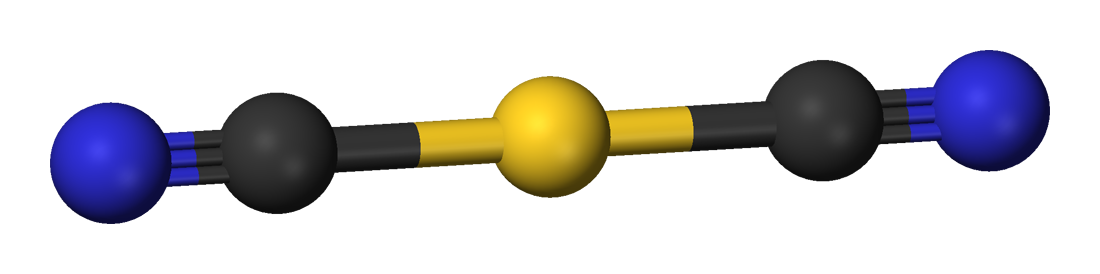 Potassium dicyanoaurate is a salt. The dicyanoaurate anion is linear according to
Potassium dicyanoaurate is a salt. The dicyanoaurate anion is linear according to
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
with formula K u(CN)2 It is a colorless to white solid that is soluble in water and slightly soluble in alcohol. The salt itself is often not isolated, but solutions of the dicyanoaurate ion ( u(CN)2sup>−) are generated on a large scale in the extraction of gold from its ores.
Production
In mining of gold from dilute sources, gold is selectively extracted by dissolution in aqueous solutions of cyanide, provided by dissolving sodium cyanide,potassium cyanide
Potassium cyanide is a compound with the formula KCN. This colorless crystalline salt, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications includ ...
and/or calcium cyanide. The reaction for the dissolution of gold, the "Elsner Equation", is:
:4 Au + 8 KCN + O2 + 2 H2O → 4 K u(CN)2+ 4 KOH
In this process, oxygen is the oxidant.
It can also be produced by reaction of gold(I) salts with excess potassium cyanide.
:AuCl + 2 KCN → K u(CN)2 + KCl
Structure
: Potassium dicyanoaurate is a salt. The dicyanoaurate anion is linear according to
Potassium dicyanoaurate is a salt. The dicyanoaurate anion is linear according to X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
. On the basis of infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, the dicyanoaurate anion adopts a very similar structure in sodium dicyanoaurate (NaAu(CN)2).
Uses
Dicyanoaurate is the soluble species that is the focus of gold cyanidation, the hydrometallurgical process for winning gold from dilute ores. In fact, sodium cyanide, not the potassium salt, is more widely used in commercial processes. Aside from its major use as an intermediate in the extraction of gold, potassium dicyanoaurate is often used in gold plating applications.Related compounds
The compound containing gold(III) cyanide is also known: potassium tetracyanoaurate(III), K u(CN)4 Its use is less common. The potassium ion can be replaced with quaternary ammonium cations as in tetrabutylammonium dicyanoaurate.Safety
The ingestion of a gram quantities of potassium dicyanoaurate has led to death.References
{{Cyanides Cyanides Aurates Gold(I) compounds Potassium compounds Cyanometallates