plutonium-244 on:
[Wikipedia]
[Google]
[Amazon]
Plutonium-244 (244Pu) is an isotope of plutonium that has a
 Plutonium-244 is one of several extinct
Plutonium-244 is one of several extinct
half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of 80 million years. This is longer than any of the other isotopes of plutonium and longer than any other actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
isotope except for the three naturally abundant ones: uranium-235 (704 million years), uranium-238 (4.468 billion years), and thorium-232 (14.05 billion years). Although studies are in conflict, given the mathematics of the decay of plutonium-244, an exceedingly small amount should still be present in the Earth's composition, making plutonium a likely although unproven candidate as the shortest lived primordial element
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
.
Natural occurrence
Accurate measurements, beginning in the early 1970s, have detectedprimordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before t ...
plutonium-244, making it the shortest-lived primordial nuclide. The amount of 244Pu in the pre- Solar nebula (4.57×109 years ago) was estimated as 0.8% the amount of 238U. As the age of the Earth
The age of Earth is estimated to be 4.54 ± 0.05 billion years This age may represent the age of Earth's accretion, or core formation, or of the material from which Earth formed. This dating is based on evidence from radiometric age-dating of m ...
is about 57 half-lives of 244Pu, the amount of plutonium-244 left should be very small; Hoffman ''et al.'' estimated its content in the rare-earth mineral bastnasite as = 1.0×10−18 g/g, which corresponded to the content in the Earth crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
as low as 3×10−25 g/g (i.e. the total mass of plutonium-244 in Earth's crust is about 9 g). Since plutonium-244 cannot be easily produced by natural neutron capture in the low neutron activity environment of uranium ores (see below), its presence cannot plausibly be explained by any other means than creation by r-process nucleosynthesis in supernovae
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
or neutron star merger
A neutron star merger is a type of stellar collision.
It occurs in a fashion similar to the rare brand of type Ia supernovae resulting from merging white dwarf stars.
When two neutron stars orbit each other closely, they gradually spiral i ...
s. Plutonium-244 thus should be the second shortest-lived (after samarium-146) and the heaviest primordial isotope yet detected or theoretically predicted.
Trace amounts of 244Pu (that arrived on Earth within the last 10 million years) were found in rock from the Pacific ocean by a Japanese oil exploration company.
The detection of primordial 244Pu in 1971 is not confirmed by recent, more sensitive measurements using the method of accelerator mass spectrometry
Accelerator mass spectrometry (AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before mass analysis. The special strength of AMS among the mass spectrometric methods is its power to separate a r ...
. In this study, no traces of plutonium-244 in the samples of bastnasite (taken from the same mine as in the early study) were observed, so only an upper limit on the 244Pu content was obtained: < 0.15×10−18 g/g, which is 370 (or less) atoms per gram of the sample, at least 7 times lower than the abundance measured by Hoffman et al.
Live interstellar plutonium-244 has been detected in meteorite dust in marine sediments, although the levels detected are much lower than would be expected from current modelling of the in-fall from the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstellar ...
. It is important to recall, however, that in order to be a primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
– one constituting the amalgam orbiting the Sun
The Sun is the star at the center of the Solar System. It is a nearly perfect ball of hot plasma, heated to incandescence by nuclear fusion reactions in its core. The Sun radiates this energy mainly as light, ultraviolet, and infrared radi ...
that ultimately coalesced into the Earth – that plutonium-244 must have comprised some of the solar nebula, rather than having been replenished by extrasolar meteoritic dust. The presence of plutonium-244 in meteoritic composition without evidence the meteor originated from the formational disc of the Solar System supports the hypothesis that 244Pu was abundant enough to have been a part of that disc, if an extrasolar meteor contained it in some other gravitationally supported system, but such a meteor cannot prove the hypothesis. Only the unlikely discovery of live 244Pu within the Earth's composition could do that.
As an extinct radionuclide
 Plutonium-244 is one of several extinct
Plutonium-244 is one of several extinct radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s that preceded the formation of the Solar System. Its half-life of 80 million years ensured its circulation across the solar system before its extinction, and indeed, 244Pu has not yet been found in matter other than meteorites. Radionuclides such as 244Pu undergo decay to produce fissiogenic (i.e., arising from fission) xenon isotopes that can then be used to time the events of the early solar system. In fact, by analyzing data from Earth's mantle which indicates that about 30% of the existing fissiogenic xenon is attributable to 244Pu decay, the timing of Earth's formation can be inferred to have occurred nearly 50–70 million years following the formation of the Solar System.
Preceding the analysis of mass spectra
A mass spectrum is a histogram plot of intensity vs. ''mass-to-charge ratio'' (''m/z'') in a chemical sample, usually acquired using an instrument called a ''mass spectrometer''. Not all mass spectra of a given substance are the same; for example ...
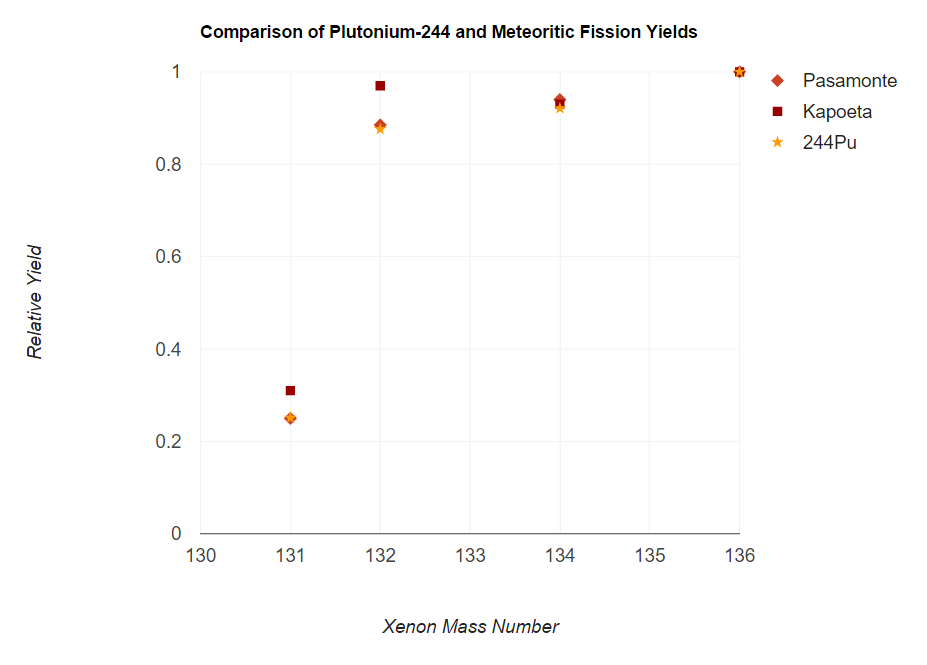
data obtained by analyzing samples found in meteorites, it was inferential at best to accredit 244Pu as being the nuclide responsible for the fissiogenic xenon found. However, an analysis of a laboratory sample of 244Pu compared with that of fissiogenic xenon gathered from the meteorites Pasamonte and Kapoeta produced matching spectra that immediately left little doubt as to the source of the isotopic xenon anomalies. Spectra data was further acquired for another actinide isotope, 244Cm, but such data proved contradictory and helped erase further doubts that the fission was appropriately attributed to 244Pu.
Both the examination of spectra data and study of fission tracks led to several findings of plutonium-244. In Western Australia, the analysis of the mass spectrum of xenon within 4.1–4.2 billion-year-old zircons was met with findings of diverse levels of 244Pu fission. Presence of 244Pu fission tracks can be established by using the initial ratio of 244Pu to 238U (Pu/U)0 at a time T0 = years, when Xe formation first began in meteorites, and by considering how the ratio of Pu/U fission tracks varies over time. Examination of a whitlockite crystal within a lunar rock specimen brought over from the Apollo 14
Apollo 14 (January 31, 1971February 9, 1971) was the eighth crewed mission in the United States Apollo program, the third to land on the Moon, and the first to land in the lunar highlands. It was the last of the " H missions", landings at s ...
mission established proportions of Pu/U fission tracks consistent with the (Pu/U)0 time dependence.
Production
Unlikeplutonium-238
Plutonium-238 (238Pu or Pu-238) is a fissile, radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 isotope suita ...
, plutonium-239, plutonium-240, plutonium-241, and plutonium-242, plutonium-244 is not produced in quantity by the nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the ''front end'', which are the preparation of the fuel, steps in the ''service period'' in w ...
, because further neutron capture on plutonium-242 produces plutonium-243 which has a short half-life (5 hours) and quickly beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For e ...
s to americium-243
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ...
before having much opportunity to further capture neutrons in any but very high neutron flux environments. The global inventory of 244Pu is roughly 20 grams. Plutonium-244 is also a minor constituent of thermonuclear
Thermonuclear fusion is the process of atomic nuclei combining or “fusing” using high temperatures to drive them close enough together for this to become possible. There are two forms of thermonuclear fusion: ''uncontrolled'', in which the re ...
fallout, with a global 244Pu/239Pu fallout ratio of (5.7 ± 1.0) × 10−5.
Applications
Plutonium-244 is used as aninternal standard An internal standard in analytical chemistry is a chemical substance that is added in a constant amount to samples, the blank and calibration standards in a chemical analysis. This substance can then be used for calibration by plotting the ratio of ...
for isotope dilution
Isotope dilution analysis is a method of determining the quantity of chemical substances. In its most simple conception, the method of isotope dilution comprises the addition of known amounts of isotopically enriched substance to the analyzed samp ...
mass spectrometry analysis of plutonium.
References
{{Isotopes of plutonium Actinides Nuclear materials Isotopes of plutonium