phosphorescent on:
[Wikipedia]
[Google]
[Amazon]

 Phosphorescence is a type of
Phosphorescence is a type of
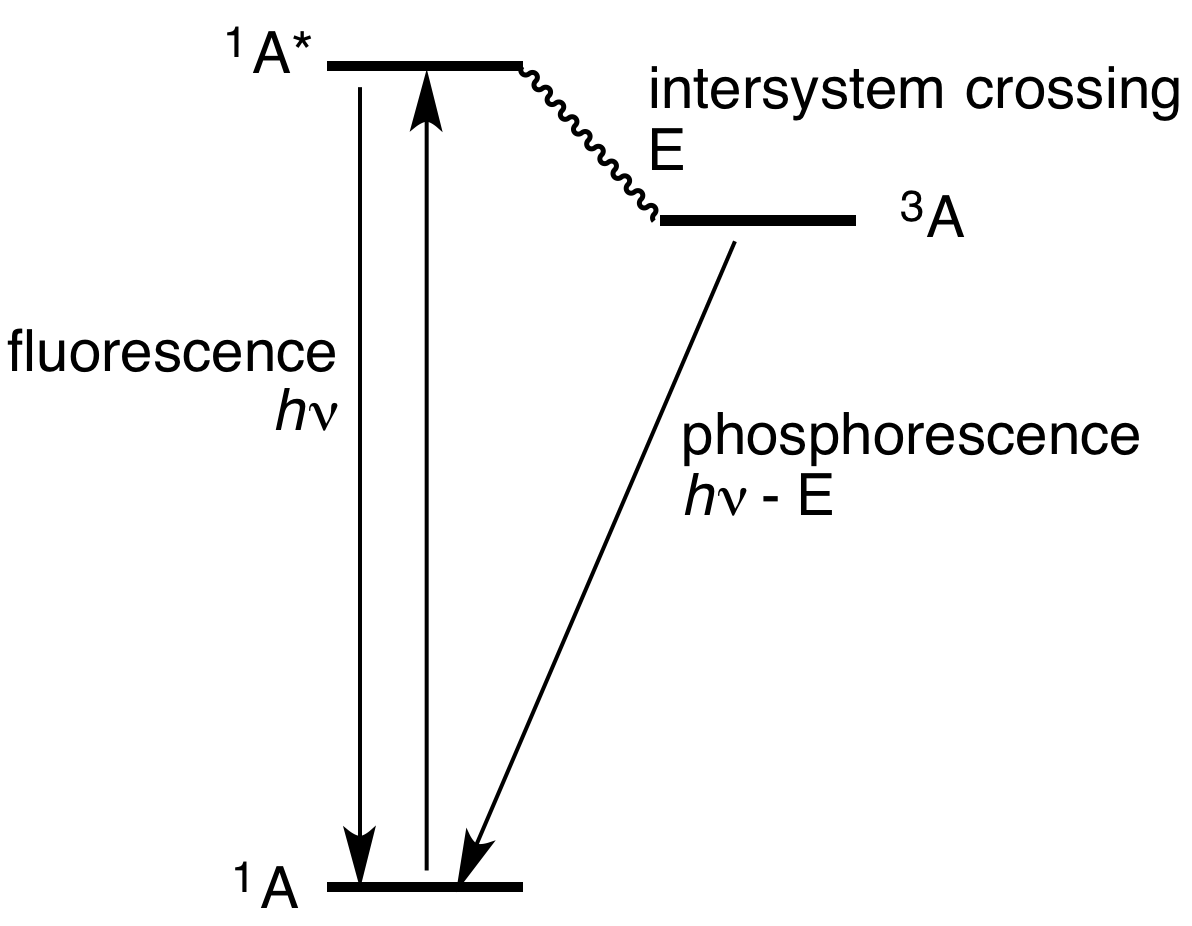 In simple terms, phosphorescence is a process in which energy absorbed by a substance is released relatively slowly in the form of light. This is in some cases the mechanism used for glow-in-the-dark materials which are "charged" by exposure to light. Unlike the relatively swift reactions in fluorescence, such as those seen in
In simple terms, phosphorescence is a process in which energy absorbed by a substance is released relatively slowly in the form of light. This is in some cases the mechanism used for glow-in-the-dark materials which are "charged" by exposure to light. Unlike the relatively swift reactions in fluorescence, such as those seen in
 Most photoluminescent events, in which a chemical substrate absorbs and then re-emits a
Most photoluminescent events, in which a chemical substrate absorbs and then re-emits a
File:Phosphorescent pigments, in light, in dark, after 4 min - zinc sulfide and strontium aluminate.jpg, Zinc sulfide (left) and strontium aluminate (right), in visible light, in darkness, and after 4 minutes in the dark.
File:Phosphorescent pigment calcium sulfide and silicate, emitting red and blue.jpg, Calcium sulfide (left) and metal-earth silicate (right) phosphoresce in red and blue respectively.
Since both phosphorescence (transition from T1 to S0) and the generation of T1 from an excited singlet state (e.g., S1) via intersystem crossing (ISC) are spin-forbidden processes, most organic materials exhibit insignificant phosphorescence as they mostly fail to populate the excited triplet state, and, even if T1 is formed, phosphorescence is most frequently outcompeted by non-radiative pathways. One strategy to enhance the ISC and phosphorescence is the incorporation of heavy atoms, which increase spin-orbit coupling (SOC). Additionally, the SOC (and therefore the ISC) can be promoted by coupling n-π* and π-π* transitions with different angular momenta, also known as
 In 1974 Becky Schroeder was given a US patent for her invention of the "Glow Sheet" which used phosphorescent lines under writing paper to help people write in low-light conditions.
Glow in the dark material is added to the plastic blend used in injection molds to make some
In 1974 Becky Schroeder was given a US patent for her invention of the "Glow Sheet" which used phosphorescent lines under writing paper to help people write in low-light conditions.
Glow in the dark material is added to the plastic blend used in injection molds to make some
File:Shadow Wall - before.png, Before image of capturing a shadow on a phosphorescent wall.
File:Shadow Wall - after.png, After image of capturing a shadow on a phosphorescent wall.

 Phosphorescence is a type of
Phosphorescence is a type of photoluminescence
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. photo ...
related to fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed.
In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light
A blacklight, also called a UV-A light, Wood's lamp, or ultraviolet light, is a lamp that emits long-wave (UV-A) ultraviolet light and very little visible light. One type of lamp has a violet filter material, either on the bulb or in a sepa ...
it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emitting light within nanoseconds (billionths of a second) after the excitation radiation is removed, phosphorescent materials may continue to emit an afterglow ranging from a few microseconds to many hours after the excitation is removed.
There are two separate mechanisms that may produce phosphorescence, called triplet phosphorescence (or simply phosphorescence) and persistent phosphorescence (or persistent luminescence
Commonly referred as phosphorescence, persistent luminescence is the emission of light by a phosphorescent material after an excitation by ultraviolet or visible light. Such materials would "glow in the dark".
Mechanism
The mechanism underlying ...
). Triplet phosphorescence occurs when an atom absorbs a high-energy photon, and the energy becomes locked in the spin multiplicity of the electrons, generally changing from a fluorescent "singlet state" to a slower emitting "triplet state". The slower timescales of the reemission are associated with " forbidden" energy state
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The ...
transitions in quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
. As these transitions occur relatively slowly in certain materials, absorbed radiation is reemitted at a lower intensity, ranging from a few microseconds to as much as one second after the excitation is removed.
On the other hand, persistent phosphorescence occurs when a high-energy photon is absorbed by an atom and its electron becomes trapped in a defect in the lattice of the crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
line or amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language, Gr ...
material. A defect such as a missing atom (vacancy defect
In crystallography, a vacancy is a type of point defect in a crystal where an atom is missing from one of the lattice sites.Ehrhart, P. (1991) "Properties and interactions of atomic defects in metals and alloys", chapter 2, p. 88 in ''Landolt-B� ...
) can trap an electron like a pitfall, storing that electron's energy until released by a random spike of thermal (vibrational) energy. Such a substance will then emit light of gradually decreasing intensity, ranging from a few seconds to up to several hours after the original excitation.
Everyday examples of phosphorescent materials are the glow-in-the-dark toys, stickers, paint and clock dials that glow after being charged with a bright light such as in any normal reading or room light. Typically, the glow slowly fades out, sometimes within a few minutes or up to a few hours in a dark room.
The study of phosphorescent materials led to the discovery of radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
.
Etymology
The term ''phosphorescence'' comes from the ancient Greek word ''φῶς'' (''phos''), meaning "light", and the Greek suffix ''-φόρος'' (''-phoros''), meaning "to bear", combined with theLatin
Latin (, or , ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through ...
suffix ''-escentem'', meaning "becoming of", "having a tendency towards", or "with the essence of". Thus, phosphorescence literally means "having a tendency to bear light". It was first recorded in 1766.
The term ''phosphor'' had been used since the Middle Ages
In the history of Europe, the Middle Ages or medieval period lasted approximately from the late 5th to the late 15th centuries, similar to the post-classical period of global history. It began with the fall of the Western Roman Empire ...
to describe minerals that glowed in the dark. One of the most famous, but not the first, was Bolognian phosphor. Around 1604, Vincenzo Casciarolo discovered a " lapis solaris" near Bologna, Italy. Once heated in an oxygen-rich furnace, it thereafter absorbed sunlight and glowed in the dark. In 1677, Hennig Brand
Hennig Brand (; c. 1630c. 1692 or c. 1710) was a German alchemist who lived and worked in Hamburg. In 1669, Brand accidentally discovered the chemical element phosphorus while searching for the "philosopher's stone", a substance which was believed ...
isolated a new element that glowed due to a chemiluminescent reaction when exposed to air, and named it "phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
".
In contrast, the term ''luminescence'' (from the Latin ''lumen'' for "light"), was coined by Eilhardt Wiedemann in 1888 as a term to refer to "light without heat", while "fluorescence" by Sir George Stokes
Sir George Gabriel Stokes, 1st Baronet, (; 13 August 1819 – 1 February 1903) was an Irish English physicist and mathematician. Born in County Sligo, Ireland, Stokes spent all of his career at the University of Cambridge, where he was the Luc ...
in 1852, when he noticed that, when exposing a solution of quinine sulfate
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cr ...
to light refracted through a prism, the solution glowed when exposed to the mysterious invisible-light (now known to be UV light) beyond the violet end of the spectrum. Stokes formed the term from a combination of fluorspar
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs sca ...
and opalescence
Opalescence refers to the optical phenomena displayed by the mineraloid gemstone opalopalescent. 2019. In Noah Webster's 1828 American Dictionary of the English Language. Retrieved January 7, 2019, from https://1828.mshaffer.com/d/word/opalesc ...
(preferring to use a mineral instead of a solution), albeit it was later discovered that fluorspar glows due to phosphorescence.
There was much confusion between the meanings of these terms throughout the late nineteenth to mid-twentieth centuries. Whereas the term "fluorescence" tended to refer to luminescence that ceased immediately (by human-eye standards) when removed from excitation, "phosphorescence" referred to virtually any substance that glowed for appreciable periods in darkness, sometimes to include even chemiluminescence (which occasionally produced substantial amounts of heat). Only after the 1950s and 1960s did advances in quantum electronics
Quantum optics is a branch of atomic, molecular, and optical physics dealing with how individual quanta of light, known as photons, interact with atoms and molecules. It includes the study of the particle-like properties of photons. Photons have ...
, spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter ...
, and laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The ...
s provide a measure to distinguish between the various processes that emit the light, although in common speech the distinctions are still often rather vague.
Introduction
 In simple terms, phosphorescence is a process in which energy absorbed by a substance is released relatively slowly in the form of light. This is in some cases the mechanism used for glow-in-the-dark materials which are "charged" by exposure to light. Unlike the relatively swift reactions in fluorescence, such as those seen in
In simple terms, phosphorescence is a process in which energy absorbed by a substance is released relatively slowly in the form of light. This is in some cases the mechanism used for glow-in-the-dark materials which are "charged" by exposure to light. Unlike the relatively swift reactions in fluorescence, such as those seen in laser medium
The active laser medium (also called gain medium or lasing medium) is the source of optical gain within a laser. The gain results from the stimulated emission of photons through electronic or molecular transitions to a lower energy state from a h ...
s like the common ruby
A ruby is a pinkish red to blood-red colored gemstone, a variety of the mineral corundum ( aluminium oxide). Ruby is one of the most popular traditional jewelry gems and is very durable. Other varieties of gem-quality corundum are called ...
, phosphorescent materials "store" absorbed energy for a longer time, as the processes required to reemit energy occur less often. However, timescale is still only a general distinction, as there are slow-emitting fluorescent materials, for example uranyl salts, and, likewise, some phosphorescent materials like zinc sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various ...
(in violet) are very fast. Scientifically, the phenomena are classified by the different mechanisms that produce the light, as materials that phosphoresce may be suitable for some purposes such as lighting, but may be completely unsuitable for others that require fluorescence, like lasers. Further blurring the lines, a substance may emit light by one, two, or all three mechanisms depending on the material and excitation conditions.
When the stored energy becomes locked in by the spin of the atomic electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s, a triplet state
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical r ...
can occur, slowing the emission of light, sometimes by several orders of magnitude. Because the atoms usually begin in a singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
of spin, favoring fluorescence, these types of phosphors typically produce both types of emission during illumination, and then a dimmer afterglow of strictly phosphorescent light typically lasting less than a second after the illumination is switched off.
Conversely, when the stored energy is due to persistent phosphorescence, an entirely different process occurs without a fluorescence precursor. When electrons become trapped within a defect in the atomic or molecular lattice, light is prevented from reemitting until the electron can escape. To escape, the electron needs a boost of thermal energy to help spring it out of the trap and back into orbit around the atom. Only then can the atom emit a photon. Thus, persistent phosphorescence is highly dependent on the temperature of the material.
Triplet phosphorescence
 Most photoluminescent events, in which a chemical substrate absorbs and then re-emits a
Most photoluminescent events, in which a chemical substrate absorbs and then re-emits a photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they alwa ...
of light, are fast, in the order of 10 nanosecond
A nanosecond (ns) is a unit of time in the International System of Units (SI) equal to one billionth of a second, that is, of a second, or 10 seconds.
The term combines the SI prefix ''nano-'' indicating a 1 billionth submultiple of an SI unit ( ...
s. Light is absorbed and emitted at these fast time scales in cases where the energy of the photons involved matches the available energy states and allowed transitions of the substrate. In the special case of phosphorescence, the electron which absorbed the photon (energy) undergoes an unusual intersystem crossing into an energy state of different (usually higher) ''spin multiplicity'' (''see term symbol In quantum mechanics, the term symbol is an abbreviated description of the (total) angular momentum quantum numbers in a multi-electron atom (however, even a single electron can be described by a term symbol). Each energy level of an atom with a giv ...
''), usually a triplet state
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical r ...
. As a result, the excited electron can become trapped in the triplet state with only "forbidden" transitions available to return to the lower energy singlet state. These transitions, although "forbidden", will still occur in quantum mechanics but are kinetically unfavored and thus progress at significantly slower time scales. Most phosphorescent compounds are still relatively fast emitters, with triplet decay-times in the order of milliseconds.
Common examples include the phosphor coatings used in fluorescent lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet, ult ...
s, where phosphorescence on the order of milliseconds or longer is useful for filling in the "off-time" between AC current
Alternating current (AC) is an electric current which periodically reverses direction and changes its magnitude continuously with time in contrast to direct current (DC) which flows only in one direction. Alternating current is the form in which ...
cycles, helping to reduce "flicker". Phosphors with faster decay times are used in applications like the pixels excited by free electrons (cathodoluminescence
Cathodoluminescence is an optical and electromagnetic phenomenon in which electrons impacting on a luminescent material such as a phosphor, cause the emission of photons which may have wavelengths in the visible spectrum. A familiar example is ...
) in cathode-ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a Phosphorescence, phosphorescent screen. The images may represent electrical waveforms (osci ...
television-sets, which are slow enough to allow the formation of a picture as the electron beam scans the screen, but fast enough to prevent the frames from blurring together. Even substances commonly associated with fluorescence may in fact be prone to phosphorescence, such as the liquid dyes found in highlighter
A highlighter is a type of writing device used to mark attention to sections of text by marking them with a vivid, translucent colour.
A typical highlighter is fluorescent yellow, colored with pyranine. Different compounds, such as rhodamines ( ...
pens, which is a common problem in liquid dye laser
A dye laser is a laser that uses an organic dye as the lasing medium, usually as a liquid solution. Compared to gases and most solid state lasing media, a dye can usually be used for a much wider range of wavelengths, often spanning 50 to 100 ...
s. The onset of phosphorescence in this case can sometimes be reduced or delayed significantly by the use of triplet-quenching agents.
Equation
where S is a singlet and T a triplet whose subscripts denote states (0 is the ground state, and 1 the excited state). Transitions can also occur to higher energy levels, but the first excited state is denoted for simplicity.Persistent phosphorescence
Solid materials typically come in two main types: crystalline and amorphous. In either case, a lattice or network ofatom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
s and molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s form. In crystals, the lattice is a very neat, uniform assembly. However, nearly all crystals have defects in the stacking sequence of these molecules and atoms. A vacancy defect
In crystallography, a vacancy is a type of point defect in a crystal where an atom is missing from one of the lattice sites.Ehrhart, P. (1991) "Properties and interactions of atomic defects in metals and alloys", chapter 2, p. 88 in ''Landolt-B� ...
, where an atom is simply missing from its place, leaving an empty "hole", is one type of defect. Sometimes atoms can move from place to place within the lattice, creating Schottky defect
A Schottky defect is an excitation of the site occupations in a crystal lattice leading to point defects named after Walter H. Schottky. In ionic crystals, this defect forms when oppositely charged ions leave their lattice sites and become inc ...
s or Frenkel defect
In crystallography, a Frenkel defect is a type of point defect in crystalline solids, named after its discoverer Yakov Frenkel. The defect forms when an atom or smaller ion (usually cation) leaves its place in the lattice, creating a vacanc ...
s. Other defects can occur from impurities in the lattice. For example, when a normal atom is substituted by a different atom of much larger or smaller size, a substitutional defect occurs, while an interstitial defect
In materials science, an interstitial defect is a type of point crystallographic defect where an atom of the same or of a different type, occupies an interstitial site in the crystal structure. When the atom is of the same type as those alread ...
occurs when a much smaller atom gets trapped in the "interstices", or the spaces between atoms. In contrast, amorphous materials have no "long-range order" (beyond the space of a few atoms in any direction), thus by definition are filled with defects.
When a defect occurs, depending on the type and material, it can create a hole, or a "trap". For example, a missing oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
atom from a zinc oxide
Zinc oxide is an inorganic compound with the formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement ...
compound creates a hole in the lattice, surrounded by unbound zinc-atoms. This creates a net force
In physics, a force is an influence that can change the motion of an object. A force can cause an object with mass to change its velocity (e.g. moving from a state of rest), i.e., to accelerate. Force can also be described intuitively as a ...
or attraction that can be measured in electron-volts. When a high-energy photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they alwa ...
strikes one of the zinc atoms, its electron absorbs the photon and is thrown out into a higher orbit. The electron may then enter the trap and be held in place (out of its normal orbit) by the attraction. To trigger the release of the energy, a random spike in thermal energy is needed of sufficient magnitude to boost the electron out of the trap and back into its normal orbit. Once in orbit, the electron's energy can drop back to normal (ground state) resulting in the release of a photon.
The release of energy in this way is a completely random process, governed mostly by the average temperature of the material versus the "depth" of the trap, or how many electron-volts it exerts. A trap that has a depth of 2.0 electron-volts would require a great amount of thermal energy (very high temperatures) to overcome the attraction, while at a depth of 0.1 electron-volts very little heat (very cold temperatures) are needed for the trap to even hold an electron. Higher temperatures may cause the faster release of energy, resulting in a brighter yet short-lived emission, while lower temperatures may produce dimmer but longer-lasting glows. Temperatures that are too hot or cold, depending on the substance, may not allow the accumulation or release of energy at all. The ideal depth of trap for persistent phosphorescence at room temperature is typically between 0.6 and 0.7 electron-volts. If the phosphorescent quantum yield The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Applications
Fluorescence spectroscopy
The fluorescence quantum yield is defined as the ratio of the numb ...
is high, that is, if the substance has a large number of traps of the correct depth, these substances will release significant amounts of light over long time scales, creating so-called "glow in the dark" materials.
Persistent phosphorescence is the mechanism of most anything commonly referred to as glow in the dark. Typical uses include toys, frisbees and balls, safety signs, paints and markings, make-ups, art and décor, and a variety of other uses.
Chemiluminescence
Some examples of glow-in-the-dark materials do not glow by phosphorescence. For example,glow sticks
A glow stick, also known as a light stick, chem light, light wand, light rod, and rave light, is a self-contained, short-term light-source. It consists of a translucent plastic tube containing isolated substances that, when combined, make light ...
glow due to a chemiluminescent process which is commonly mistaken for phosphorescence. In chemiluminescence, an excited state is created via a chemical reaction. The light emission tracks the kinetic progress of the underlying chemical reaction. The excited state will then transfer to a dye molecule, also known as a sensitizer or fluorophor, and subsequently fluoresce back to the ground state.
Materials
Common pigments used in phosphorescent materials includezinc sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various ...
and strontium aluminate. Use of zinc sulfide for safety related products dates back to the 1930s.
The development of strontium aluminate pigments in 1993 was spurred on by the need to find a substitute for glow-in-the-dark materials with high luminance and long phosphorescence, especially those that used promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
. This led to the discovery by Yasumitsu Aoki (Nemoto & Co.) of materials with luminance approximately 10 times greater than zinc sulfide and phosphorescence approximately 10 times longer. This has relegated most zinc sulfide based products to the novelty category. Strontium aluminate based pigments are now used in exit signs, pathway marking, and other safety related signage.
Mostafa El-Sayed
Mostafa A. El-Sayed (Arabic: مصطفى السيد) is an Egyptian-American physical chemist, a leading nanoscience researcher, a member of the National Academy of Sciences and a US National Medal of Science laureate. He was the editor-in-chief ...
's rule. Such transitions are typically exhibited by carbonyl or triazine derivatives, and most organic room-temperature phosphorescent (ORTP) materials incorporate such moieties. In turn, to inhibit competitive non-radiative deactivation pathways, including vibrational relaxation and oxygen quenching and triplet-triplet annihilations, organic phosphors have to be embedded in rigid matrices such as polymers, and molecular solids (crystals, covalent organic frameworks, and others).
Uses
 In 1974 Becky Schroeder was given a US patent for her invention of the "Glow Sheet" which used phosphorescent lines under writing paper to help people write in low-light conditions.
Glow in the dark material is added to the plastic blend used in injection molds to make some
In 1974 Becky Schroeder was given a US patent for her invention of the "Glow Sheet" which used phosphorescent lines under writing paper to help people write in low-light conditions.
Glow in the dark material is added to the plastic blend used in injection molds to make some disc golf
Disc golf, also known as frisbee golf, is a flying disc sport in which players throw a disc at a target; it is played using rules similar to golf. Most disc golf discs are made out of polypropylene plastic, otherwise known as polypropene, which ...
discs, which allow the game to be played at night.
Often clock faces of watches are painted with phosphorencent colours. Therefore, they can be used in absolute dark environments for several hours after having been exposed to bright light.
A common use of Phosphorescence is decoration. Stars made of glow-in-the-dark plastic are placed on walls, ceilings, or hanging from strings make a room look like the night sky. Other objects like figurines, cups, posters, lamp fixtures, toys & bracelet beads may also glow. Using blacklight
A blacklight, also called a UV-A light, Wood's lamp, or ultraviolet light, is a lamp that emits long-wave (UV-A) ultraviolet light and very little visible light. One type of lamp has a violet filter material, either on the bulb or in a sepa ...
s makes these things glow brightly, common at raves, bedrooms, theme parks & festivals.
Shadow wall
A shadow wall is created when a light flashes upon a person or object in front of a phosphorescent screen which temporarily captures the shadow. The screen or wall is painted with a glow-in-the-dark product that contains phosphorescent compounds. Publicly, these shadow walls can be found at certain science museums.See also
* Luminous gemstones *Luminous paint
Luminous paint or luminescent paint is paint that exhibits luminescence. In other words, it gives off visible light through fluorescence, phosphorescence, or radioluminescence. There are three types of luminous paints: fluorescent paint, ph ...
* Microsphere
Microparticles are particles between 0.1 and 100 μm in size. Commercially available microparticles are available in a wide variety of materials, including ceramics, glass, polymers, and metals. Microparticles encountered in daily life incl ...
* Persistent luminescence
Commonly referred as phosphorescence, persistent luminescence is the emission of light by a phosphorescent material after an excitation by ultraviolet or visible light. Such materials would "glow in the dark".
Mechanism
The mechanism underlying ...
* Phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or v ...
* Phosphoroscope
* Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of ...
References
External links
{{Authority control Luminescence Phosphors and scintillators Spectroscopy