Osmotic on:
[Wikipedia]
[Google]
[Amazon]
 Osmosis (, ) is the spontaneous net movement or
Osmosis (, ) is the spontaneous net movement or
link
pp. 115
and 126. * The intermediate word "osmose" and the word "osmotic" were coined by Scottish chemist Thomas Graham. See: Thomas Graham (1854
"VII. The Bakerian Lecture – On Osmotic Force,"
''Philosophical Transactions of the Royal Society (London)'', vol. 144, pp. 177–288; see especially pp. 177, 178, and 227. See also: Thomas Graham and Henry Watts, ''Elements of Chemistry: Including the Applications of the Sciences in the Arts'', 2nd ed. (London, England: Hippolyte Bailliere, 1858), vol. 2
p. 616
* The word "osmosis" first appeared in: Jabez Hogg, ''The Microscope: Its History, Construction, and Application...'', 6th ed. (London, England: George Routledge and Sons, 1867)
p. 226
* The etymology of the word "osmosis" is discussed in: In 1867, Moritz Traube invented highly selective precipitation membranes, advancing the art and technique of measurement of osmotic flow.
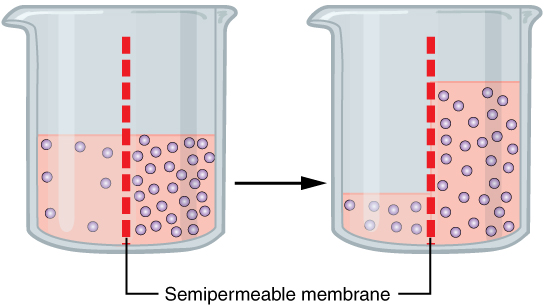
 When the membrane has a volume of pure water on both sides, water molecules pass in and out in each direction at exactly the same rate. There is no net flow of water through the membrane.
Osmosis can be demonstrated when potato slices are added to a high salt solution. The water from inside the potato moves out to the solution, causing the potato to shrink and to lose its 'turgor pressure'. The more concentrated the salt solution, the bigger the loss in size and weight of the potato slice.
Chemical gardens demonstrate the effect of osmosis in inorganic chemistry.
When the membrane has a volume of pure water on both sides, water molecules pass in and out in each direction at exactly the same rate. There is no net flow of water through the membrane.
Osmosis can be demonstrated when potato slices are added to a high salt solution. The water from inside the potato moves out to the solution, causing the potato to shrink and to lose its 'turgor pressure'. The more concentrated the salt solution, the bigger the loss in size and weight of the potato slice.
Chemical gardens demonstrate the effect of osmosis in inorganic chemistry.
Osmosis simulation in JavaAn Osmosis Experiment
{{Authority control Diffusion Water technology Membrane technology
 Osmosis (, ) is the spontaneous net movement or
Osmosis (, ) is the spontaneous net movement or diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemica ...
of solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent ...
concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work
Work may refer to:
* Work (human activity), intentional activity people perform to support themselves, others, or the community
** Manual labour, physical work done by humans
** House work, housework, or homemaking
** Working animal, an animal t ...
. Osmotic pressure is defined as the external pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of sol ...
of the solute but not on its identity.
Osmosis is a vital process in biological systems, as biological membranes are semipermeable. In general, these membranes are impermeable to large and polar molecules, such as ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
, proteins, and polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with w ...
, while being permeable to non-polar or hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
molecules like lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include ...
as well as to small molecules like oxygen, carbon dioxide, nitrogen, and nitric oxide. Permeability depends on solubility, charge, or chemistry, as well as solute size. Water molecules travel through the plasma membrane, tonoplast membrane (vacuole) or organelle membranes by diffusing across the phospholipid bilayer via aquaporin
Aquaporins, also called water channels, are channel proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells. The cell membranes of a ...
s (small transmembrane proteins similar to those responsible for facilitated diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemica ...
and ion channels). Osmosis provides the primary means by which water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
is transported into and out of cells. The turgor
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall.
It is also called ''hydrostatic pressure'', and is defined as the pressure in a fluid measured at a certain point within itself when at equilibri ...
pressure of a cell is largely maintained by osmosis across the cell membrane between the cell interior and its relatively hypotonic environment.
History
Some kinds of osmotic flow have been observed since ancient times, e.g., on the construction of Egyptian pyramids.Hammel, H.T.; Scholander, P.F. (1976). Perspectives on the Mechanism of Osmosis and Imbibition In: ''Osmosis and tensile solvent''. Springer-Verlag, Berlin, Heidelberg, New Yorklink
Jean-Antoine Nollet
Jean-Antoine Nollet (; 19 November 170025 April 1770) was a French clergyman and physicist who did a number of experiments with electricity and discovered osmosis. As a deacon in the Catholic Church, he was also known as Abbé Nollet.
Biography ...
first documented observation of osmosis in 1748. The word "osmosis" descends from the words "endosmose" and "exosmose", which were coined by French physician René Joachim Henri Dutrochet (1776–1847) from the Greek words ἔνδον (''éndon'' "within"), ἔξω (''éxō'' "outer, external"), and ὠσμός (''ōsmós'' "push, impulsion").Etymology of "osmosis" :
* Henri Dutrochet, ''L'Agent Immédiat du Movement Vital Dévoilé dans sa Nature et dans son Mode d'Action chez les Végétaux et chez les Animaux'' he immediate agent of living movement, its nature and mode of action revealed in plants and animals(Paris, France: Dentu, 1826)pp. 115
and 126. * The intermediate word "osmose" and the word "osmotic" were coined by Scottish chemist Thomas Graham. See: Thomas Graham (1854
"VII. The Bakerian Lecture – On Osmotic Force,"
''Philosophical Transactions of the Royal Society (London)'', vol. 144, pp. 177–288; see especially pp. 177, 178, and 227. See also: Thomas Graham and Henry Watts, ''Elements of Chemistry: Including the Applications of the Sciences in the Arts'', 2nd ed. (London, England: Hippolyte Bailliere, 1858), vol. 2
p. 616
* The word "osmosis" first appeared in: Jabez Hogg, ''The Microscope: Its History, Construction, and Application...'', 6th ed. (London, England: George Routledge and Sons, 1867)
p. 226
* The etymology of the word "osmosis" is discussed in: In 1867, Moritz Traube invented highly selective precipitation membranes, advancing the art and technique of measurement of osmotic flow.
Description
Osmosis is the movement of a solvent across a semipermeable membrane toward a higher concentration of solute. In biological systems, the solvent is typically water, but osmosis can occur in other liquids, supercritical liquids, and even gases. When a cell is submerged inwater
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, the water molecules pass through the cell membrane from an area of low solute concentration to high solute concentration. For example, if the cell is submerged in saltwater, water molecules move out of the cell. If a cell is submerged in freshwater, water molecules move into the cell. Mechanism
The mechanism responsible for driving osmosis has commonly been represented in biology and chemistry texts as either the dilution of water by solute (resulting in lower concentration of water on the higher solute concentration side of the membrane and therefore adiffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemica ...
of water along a concentration gradient) or by a solute's attraction to water (resulting in less free water on the higher solute concentration side of the membrane and therefore net movement of water toward the solute). Both of these notions have been conclusively refuted.
The diffusion model of osmosis is rendered untenable by the fact that osmosis can drive water across a membrane toward a higher concentration of water. The "bound water" model is refuted by the fact that osmosis is independent of the size of the solute molecules—a colligative property—or how hydrophilic they are.
It is difficult to describe osmosis without a mechanical or thermodynamic explanation, but essentially there is an interaction between the solute and water that counteracts the pressure that otherwise free solute molecules would exert. One fact to take note of is that heat from the surroundings is able to be converted into mechanical energy (water rising).
Many thermodynamic explanations go into the concept of chemical potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species ...
and how the function of the water on the solution side differs from that of pure water due to the higher pressure and the presence of the solute counteracting such that the chemical potential remains unchanged. The virial theorem
In mechanics, the virial theorem provides a general equation that relates the average over time of the total kinetic energy of a stable system of discrete particles, bound by potential forces, with that of the total potential energy of the system. ...
demonstrates that attraction between the molecules (water and solute) reduces the pressure, and thus the pressure exerted by water molecules on each other in solution is less than in pure water, allowing pure water to "force" the solution until the pressure reaches equilibrium.
Role in living things
Osmotic pressure is the main agent of support in many plants. The osmotic entry of water raises the turgor pressure exerted against the cell wall, until it equals the osmotic pressure, creating asteady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ''p' ...
.
When a plant cell is placed in a solution that is hypertonic relative to the cytoplasm, water moves out of the cell and the cell shrinks. In doing so, the cell becomes ''flaccid''. In extreme cases, the cell becomes plasmolyzed – the cell membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment ( ...
disengages with the cell wall due to lack of water pressure on it.
When a plant cell is placed in a solution that is hypotonic relative to the cytoplasm, water moves into the cell and the cell swells to become ''turgid''.
Osmosis is responsible for the ability of plant roots to draw water from the soil. Plants concentrate solutes in their root cells by active transport
In cellular biology, ''active transport'' is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellul ...
, and water enters the roots by osmosis. Osmosis is also responsible for controlling the movement of guard cells.
In unusual environments, osmosis can be very harmful to organisms. For example, freshwater and saltwater aquarium fish placed in water of a different salinity than that to which they are adapted to will die quickly, and in the case of saltwater fish, dramatically. Another example of a harmful osmotic effect is the use of table salt to kill leeches and slugs.
Suppose an animal or a plant cell is placed in a solution of sugar or salt in water.
* If the medium is ''hypotonic'' relative to the cell cytoplasm, the cell will gain water through osmosis.
* If the medium is ''isotonic'', there will be no net movement of water across the cell membrane.
* If the medium is ''hypertonic'' relative to the cell cytoplasm, the cell will lose water by osmosis.
This means that if a cell is put in a solution which has a solute concentration higher than its own, it will shrivel, and if it is put in a solution with a lower solute concentration than its own, the cell will swell and may even burst.
Factors
Osmotic pressure
Osmosis may be opposed by increasing the pressure in the region of high solute concentration with respect to that in the low solute concentration region. The force per unit area, or pressure, required to prevent the passage ofwater
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
(or any other high-liquidity
Liquidity is a concept in economics involving the convertibility of assets and obligations. It can include:
* Market liquidity, the ease with which an asset can be sold
* Accounting liquidity, the ability to meet cash obligations when due
* Liq ...
solution) through a selectively permeable membrane and into a solution of greater concentration is equivalent to the osmotic pressure of the solution, or turgor
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall.
It is also called ''hydrostatic pressure'', and is defined as the pressure in a fluid measured at a certain point within itself when at equilibri ...
. Osmotic pressure is a colligative property, meaning that the property depends on the concentration of the solute, but not on its content or chemical identity.
Osmotic gradient
The osmotic gradient is the difference in concentration between two solutions on either side of a semipermeable membrane, and is used to tell the difference in percentages of the concentration of a specific particle dissolved in a solution. Usually the osmotic gradient is used while comparing solutions that have a semipermeable membrane between them allowing water to diffuse between the two solutions, toward the hypertonic solution (the solution with the higher concentration). Eventually, the force of the column of water on the hypertonic side of the semipermeable membrane will equal the force of diffusion on the hypotonic (the side with a lesser concentration) side, creating equilibrium. When equilibrium is reached, water continues to flow, but it flows both ways in equal amounts as well as force, therefore stabilizing the solution.Variation
Reverse osmosis
Reverse osmosis is a separation process that uses pressure to force a solvent through asemi-permeable membrane
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecule ...
that retains the solute on one side and allows the pure solvent to pass to the other side, forcing it from a region of high solute concentration through a membrane to a region of low solute concentration by applying a pressure in excess of the osmotic pressure.
Forward osmosis
Osmosis may be used directly to achieve separation of water from a solution containing unwanted solutes. A "draw" solution of higher osmotic pressure than the feed solution is used to induce a net flow of water through a semi-permeable membrane, such that the feed solution becomes concentrated as the draw solution becomes dilute. The diluted draw solution may then be used directly (as with an ingestible solute like glucose), or sent to a secondary separation process for the removal of the draw solute. This secondary separation can be more efficient than a reverse osmosis process would be alone, depending on the draw solute used and the feedwater treated. Forward osmosis is an area of ongoing research, focusing on applications in desalination,water purification
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for hu ...
, water treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, inc ...
, food processing, and other areas of study.
See also
* Brining *Homeostasis
In biology, homeostasis (British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and ...
*Osmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
* Osmotic shock
*Osmotic power
Osmotic power, salinity gradient power or blue energy is the energy available from the difference in the salt concentration between seawater and river water. Two practical methods for this are reverse electrodialysis (RED) and
pressure retarde ...
*Plasmolysis
Plasmolysis is the process in which cells lose water in a hypertonic solution. The reverse process, deplasmolysis or cytolysis, can occur if the cell is in a hypotonic solution resulting in a lower external osmotic pressure and a net flow of wate ...
* Reverse osmosis plant
*Salinity gradient power
Osmotic power, salinity gradient power or blue energy is the energy available from the difference in the salt concentration between seawater and river water. Two practical methods for this are reverse electrodialysis (RED) and
pressure retarde ...
* Water potential
References
External links
Osmosis simulation in Java
{{Authority control Diffusion Water technology Membrane technology