orthoester on:
[Wikipedia]
[Google]
[Amazon]
 In
In

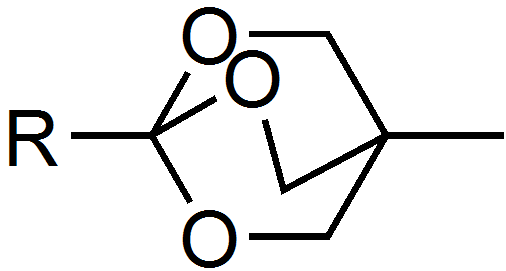 Trimethyl orthoformate and triethylorthoacetate are reagents. Another example is the bicyclic OBO protecting group (4-methyl-2,6,7-trioxa-bicyclo[2.2.2]octan-1-yl) which is formed by the action of (3-methyloxetan-3-yl)methanol on activated carboxylic acids in the presence of Lewis acids. The group is base stable and can be cleaved in two steps under mild conditions, mildly acidic hydrolysis yields the ester of tris(hydroxymethyl)ethane which is then cleaved using e.g. an aqueous carbonate solution.
Trimethyl orthoformate and triethylorthoacetate are reagents. Another example is the bicyclic OBO protecting group (4-methyl-2,6,7-trioxa-bicyclo[2.2.2]octan-1-yl) which is formed by the action of (3-methyloxetan-3-yl)methanol on activated carboxylic acids in the presence of Lewis acids. The group is base stable and can be cleaved in two steps under mild conditions, mildly acidic hydrolysis yields the ester of tris(hydroxymethyl)ethane which is then cleaved using e.g. an aqueous carbonate solution.
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J. ...
, an ortho ester is a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
containing three alkoxy group
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also ...
s attached to one carbon atom, i.e. with the general formula . Orthoesters may be considered as products of exhaustive alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
of unstable orthocarboxylic acids and it is from these that the name 'ortho ester' is derived. An example is ethyl orthoacetate
Triethyl orthoacetate is the organic compound with the formula CH3C(OC2H5)3. It is the ethyl orthoester of acetic acid. It is a colorless oily liquid.
Triethyl orthoacetate is used in organic synthesis for acetylation
:
In organic chemistry, ...
, , more correctly known as 1,1,1-triethoxyethane.
Synthesis
Ortho esters can be prepared by thePinner reaction
The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt (alkyl imidate salt); this is sometimes referred to as a Pinner salt.
The reaction is named after Adolf Pinner, who first described ...
, in which nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
s react with alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of se ...
s in the presence of one equivalent of hydrogen chloride. The reaction proceeds by formation of imido ester hydrochloride:
:RCN + R′OH + HCl → C(OR′)=NH2sup>+Cl−
Upon standing in the presence of excess alcohol, this intermediate converts to the ortho ester:
: C(OR′)=NH2sup>+Cl− + 2R′OH → RC(OR′)3 + NH4Cl
The reaction requires anhydrous conditions.
Although a less common method, ortho esters were first produced by reaction of 1,1,1-trichloroalkanes with sodium alkoxide:
:RCCl3 + 3NaOR′ → RC(OR′)3 + 3NaCl
Reactions
Hydrolysis
Ortho esters are readilyhydrolyzed
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
in mild aqueous acid to form ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glyceride ...
s:
: RC(OR′)3 + H2O → RCO2R′ + 2 R′OH
For example, trimethyl orthoformate
Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers. The product of reaction of an aldeh ...
CH(OCH3)3 may be hydrolyzed (under acidic conditions) to methyl formate
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other co ...
and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
; and may be further hydrolyzed (under alkaline conditions) to salts of formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
and methanol.
:
Johnson–Claisen rearrangement
The Johnson–Claisen rearrangement is the reaction of anallyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
ic alcohol with an ortho ester containing a deprotonatable alpha carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the us ...
(e.g. triethyl orthoacetate
Triethyl orthoacetate is the organic compound with the formula CH3C(OC2H5)3. It is the ethyl orthoester of acetic acid. It is a colorless oily liquid.
Triethyl orthoacetate is used in organic synthesis for acetylation
:
In organic chemistry, ...
) to give a ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glyceride ...
.
:
Bodroux–Chichibabin aldehyde synthesis
In the Bodroux–Chichibabin aldehyde synthesis an ortho ester reacts with aGrignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
to form an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
; this is an example of a formylation reaction
A formylation reaction in organic chemistry refers to organic reactions in which an organic compound is functionalized with a formyl group (-CH=O). The reaction is a route to aldehydes (''C''-CH=O), formamides (''N''-CH=O), and formate esters (' ...
.
:
Examples
: Hygromycin_B,_an_antibiotic,_is_one_of_several_naturally_occurring_ortho_esters..html" ;"title="antibiotic.html" ;"title="Hygromycin B, an antibiotic">Hygromycin B, an antibiotic, is one of several naturally occurring ortho esters.">antibiotic.html" ;"title="Hygromycin B, an antibiotic">Hygromycin B, an antibiotic, is one of several naturally occurring ortho esters. Trimethyl orthoformate and triethylorthoacetate are reagents. Another example is the bicyclic OBO protecting group (4-methyl-2,6,7-trioxa-bicyclo[2.2.2]octan-1-yl) which is formed by the action of (3-methyloxetan-3-yl)methanol on activated carboxylic acids in the presence of Lewis acids. The group is base stable and can be cleaved in two steps under mild conditions, mildly acidic hydrolysis yields the ester of tris(hydroxymethyl)ethane which is then cleaved using e.g. an aqueous carbonate solution.
Trimethyl orthoformate and triethylorthoacetate are reagents. Another example is the bicyclic OBO protecting group (4-methyl-2,6,7-trioxa-bicyclo[2.2.2]octan-1-yl) which is formed by the action of (3-methyloxetan-3-yl)methanol on activated carboxylic acids in the presence of Lewis acids. The group is base stable and can be cleaved in two steps under mild conditions, mildly acidic hydrolysis yields the ester of tris(hydroxymethyl)ethane which is then cleaved using e.g. an aqueous carbonate solution.
See also
*Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments no ...
, C(OR)2R2
* Orthocarbonate, C(OR)4.
References
{{Reflist Functional groups