Organoaluminium on:
[Wikipedia]
[Google]
[Amazon]
 Organoaluminium chemistry is the study of compounds containing bonds between
Organoaluminium chemistry is the study of compounds containing bonds between

 For terminal alkynes, the reaction generally proceeds with good regioselectivity (>90:10 rr) and complete ''syn'' selectivity, even in the presence of propargylic or homopropargylic heteroatom substituents. Unfortunately, extension of the zirconocene-catalyzed methylalumination to alkylalumination with higher alkyls results in lower yields and poor regioselectivities.
For terminal alkynes, the reaction generally proceeds with good regioselectivity (>90:10 rr) and complete ''syn'' selectivity, even in the presence of propargylic or homopropargylic heteroatom substituents. Unfortunately, extension of the zirconocene-catalyzed methylalumination to alkylalumination with higher alkyls results in lower yields and poor regioselectivities.
 Organoaluminium chemistry is the study of compounds containing bonds between
Organoaluminium chemistry is the study of compounds containing bonds between carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
and aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industriall ...
, the monomer triisobutylaluminium
Triisobutylaluminium (TiBA) is an organoaluminium compound with the formula Al(CH2CH(CH3)2)3. This colorless pyrophoric liquid is mainly used to make linear primary alcohols and α-olefins.Michael J. Krause, Frank Orlandi, Alfred T. Saurage, Jos ...
, and the titanium-aluminium compound called Tebbe's reagent
Tebbe's reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It ...
. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any s ...
of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
History
The first organoaluminium compound (C2H5)3Al2I3 was discovered in 1859. Organoaluminium compounds were, however, little known until the 1950s whenKarl Ziegler
Karl Waldemar Ziegler (26 November 1898 – 12 August 1973) was a German chemist who won the Nobel Prize in Chemistry in 1963, with Giulio Natta, for work on polymers. The Nobel Committee recognized his "excellent work on organometallic compound ...
and colleagues discovered the direct synthesis of trialkylaluminium compounds and applied these compounds to catalytic olefin polymerization. This line of research ultimately resulted in the Nobel Prize to Ziegler.
Structure and bonding
Aluminium(III) compounds
Organoaluminium compounds generally feature three- and four-coordinate Al centers, although highercoordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central io ...
s are observed with inorganic ligands such as fluoride. In accord with the usual trends, four-coordinate Al prefers to be tetrahedral. In contrast to boron, aluminium is a larger atom and easily accommodates four carbon ligands. The triorganoaluminium compounds are thus usually dimeric with a pair of bridging alkyl ligands, e.g., Al2(C2H5)4(μ-C2H5)2. Thus, despite its common name of triethylaluminium, this compound contains two aluminium centres, and six ethyl groups. When the organoaluminium compound contain hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
or halide, these smaller ligands tend to occupy the bridging sites. Three coordination occurs when the R groups is bulky, e.g. Al(Mes)3 (Mes = 2,4,6-Me3C6H2 or mesityl) or isobutyl.

Ligand exchange in trialkylaluminium compounds
The trialkylaluminium dimers often participate in dynamic equilibria, resulting in the interchange of bridging and terminal ligands as well as ligand exchange between dimers. Even in noncoordinatingsolvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s, Al-Me exchange is fast, as confirmed by proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the struct ...
spectroscopy. For example, at −25 °C the 1H NMR spectrum of Me6Al2 comprises two signals in 1:2 ratio, as expected from the solid state structure. At 20 °C, only one signal is observed because exchange of terminal and bridging methyl groups is too fast to be resolved by NMR. The high Lewis acidity of the monomeric species is related to the size of the Al(III) center and its tendency to achieve an octet configuration.
Low oxidation state organoaluminium compounds
The first organoaluminium compound with an Al-Al bond was reported in 1988 as (((Me3Si)2CH)2Al)2 (a dialane). They are typically prepared reduction of the dialkylaluminium chlorides by metallic potassium: :(R2AlCl)2 + 2 K → R2Al-AlR2 + 2 KCl Another notable group of alanes are tetraalanes containing four Al(I) centres. These compounds adopt atetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have be ...
core, as illustrated by ( Cp*Al)4 and ((Me3Si3C)Al)4. The cluster i-Bu)12.html" ;"title="isobutyl.html" ;"title="l12(isobutyl">i-Bu)12">isobutyl.html" ;"title="l12(isobutyl">i-Bu)12sup>2− was obtained from related investigations on the reduction of organoaluminium compounds. This dianion adopts an icosahedral structure reminiscent of dodecaborate ([B12H12]2−). Its formal oxidation state is less than one.
Preparation
From alkyl halides and aluminium
Industrially, simple aluminium alkyls of the type Al2R6 (R = Me, Et) are prepared in a two-step process beginning with thealkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecti ...
of aluminium powder:
:2 Al + 3 CH3CH2Cl → (CH3CH2)3Al2Cl3
The reaction resembles the synthesis Grignard reagents. The product, (CH3CH2)3Al2Cl3, is called ethylaluminium sesquichloride. The term sesquichloride refers to the fact that, on average, the Cl:Al ratio is 1.5. These sesquichlorides can be converted to the triorganoaluminium derivatives by reduction:
:2 (CH3CH2)3Al2Cl3 + 6 Na → (CH3CH2)6Al2 + 2 Al + 6 NaCl
This method is used for production of trimethylaluminium and triethylaluminium
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( C2H5)6 (abbreviated as Al2Et6 or TEA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially ...
.
Hydroalumination
Aluminium powder reacts directly with certain terminal alkenes in the presence of hydrogen. The process entails two steps, the first producing dialkylaluminium hydrides. Such reactions are typically conducted at elevated temperatures and require activation by trialkylaluminium reagents: :6 Al + 3 H2 + 12 CH2=CHR → 2 Al(CH2CHR)2sub>3 For nonbulky R groups, the organoaluminium hydrides are typically trimeric. In a subsequent step, these hydrides are treated with more alkene to effect hydroalumiunation: :2 Al(CH2CHR)2sub>3 + 3 CH2=CHR → 3 l2(CH2CHR)3 Diisobutylaluminium_hydride,_which_is_dimeric,_is_prepared_by_hydride_elimination_from_triisobutylaluminium: :2_''i''-Bu3Al_→_(''i''-Bu2AlH)2_+_2_isobutene.html" ;"title="Diisobutylaluminium_hydride.html" ;"title="l2(CH2CHR)3 Diisobutylaluminium hydride">l2(CH2CHR)3 Diisobutylaluminium hydride, which is dimeric, is prepared by hydride elimination from triisobutylaluminium: :2 ''i''-Bu3Al → (''i''-Bu2AlH)2 + 2 isobutene">(CH3)2C=CH2Carboalumination
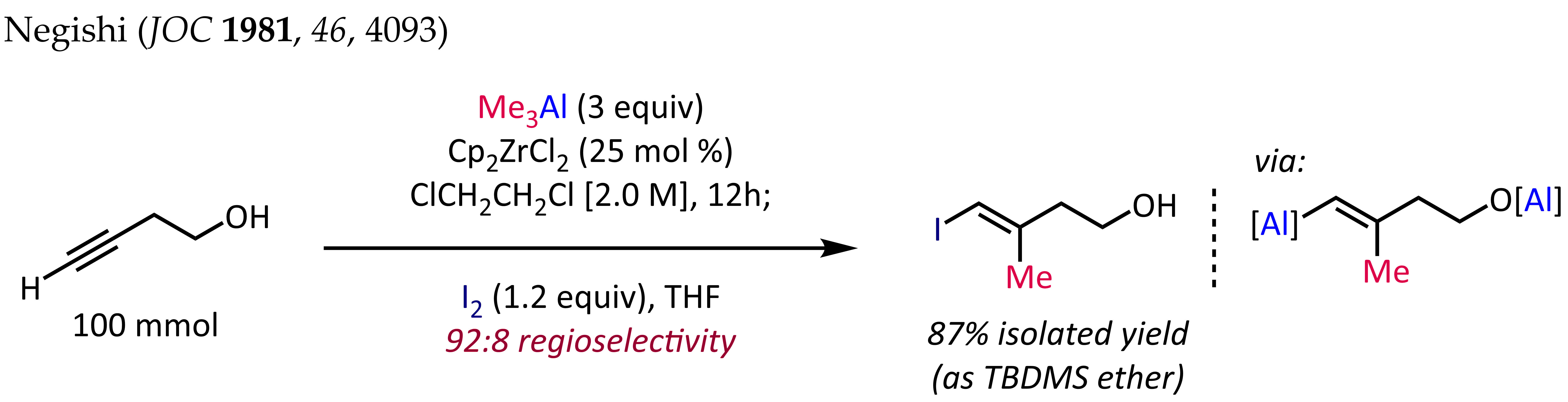
Organoaluminum compounds can react with alkenes and alkynes, resulting in the net addition of one organyl group and the metal fragment across the multiple bond (carboalumination). This process can proceed in a purely thermal manner or in the presence of a transition metal catalyst. For the uncatalyzed process, monoaddition is only possible when the alkene is substituted. For ethylene, carboalumination leads to a Poisson distribution of higher alkylaluminum species. The reaction is regioselective for 1-alkenes. The so-called ZACA reaction first reported by Ei-ichi Negishi is an example of an asymmetric carboalumination of alkenes catalyzed by a chiral zirconocene catalyst. The methylalumination of alkynes in the presence of Cp2ZrCl2 is employed for the synthesis of stereodefined trisubstituted olefin fragments, a common substructure in terpene and polyketide natural products. The synthesis of (''E'')-4-iodo-3-methylbut-3-en-1-ol shown below is a typical application of this reaction: For terminal alkynes, the reaction generally proceeds with good regioselectivity (>90:10 rr) and complete ''syn'' selectivity, even in the presence of propargylic or homopropargylic heteroatom substituents. Unfortunately, extension of the zirconocene-catalyzed methylalumination to alkylalumination with higher alkyls results in lower yields and poor regioselectivities.
For terminal alkynes, the reaction generally proceeds with good regioselectivity (>90:10 rr) and complete ''syn'' selectivity, even in the presence of propargylic or homopropargylic heteroatom substituents. Unfortunately, extension of the zirconocene-catalyzed methylalumination to alkylalumination with higher alkyls results in lower yields and poor regioselectivities.
Laboratory preparations
Although the simple members are commercially available at low cost, many methods have been developed for their synthesis in the laboratory, including metathesis or transmetalation. *Metathesis of aluminium trichloride with RLi or RMgX gives the trialkyl: :AlCl3 + 3 BuLi → Bu3Al + 3 LiCl *Transmetalation: :2 Al + 3 HgPh2 → 2 AlPh3 + 3 HgReactions
The high reactivity of organoaluminium compounds toward electrophiles is attributed to the charge separation betweenaluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
and carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atom.
Lewis acidity
Organoaluminium compounds are hard acids and readily form adducts with bases such aspyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ...
, THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
and tertiary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
s. These adducts are tetrahedral at Al.
Electrophiles
The Al–C bond is polarized such that the carbon is highly basic. Acids react to give alkanes. For example, alcohols give alkoxides: :AlR'3 + ROH → 1/n (R'2Al−OR)n + R'H A wide variety of acids can be employed beyond the simple mineral acids. Amines give amido derivatives. Withcarbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
, trialkylaluminium compounds give the dialkylaluminium carboxylate, and subsequently alkyl aluminium dicarboxylates:
:AlR3 + CO2 → R2AlO2CR
:R2AlO2CR + CO2 → RAl(O2CR)2
The conversion is reminiscent of the carbonation of Grignard reagents.
Similarly, the reaction between trialkylaluminum compounds and carbon dioxide has been used to synthesise alcohols, olefins, or ketones.
With oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
one obtains the corresponding alkoxides, which can be hydrolysed to the alcohols:
:AlR3 + 3/2 O2 → Al(OR)3
A structurally characterized organoaluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
is l(R)-O-O-CMe3 =CH(SiMe3)2
The reaction between pure trialalkylaluminum compounds and water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
, alcohols, phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
s, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s, carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
, sulfur oxide
Sulfur oxide refers to many types of sulfur and oxygen containing compounds such as SO, SO2, SO3, S7O2, S6O2, S2O2, etc.
Sulfur oxide (SO''x'') refers to one or more of the following:
* Lower sulfur oxides (S''n''O, S7O2 and S6O2)
* Sulfur mono ...
s, nitrogen oxides, halogens, and halogenated hydrocarbons
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
can be violent.
Applications
Organoaluminium compounds are widely used in the production of alkenes, alcohols, and polymers. Some relevant processes include theZiegler Process In organic chemistry, the Ziegler process (also called the Ziegler-Alfol synthesis) is a method for producing fatty alcohols from ethylene using an organoaluminium compound. The reaction produces linear primary alcohols with an even numbered carbon ...
for the production of alcohols from ethylene. Several technologies exist for the oligomerization of ethylene to give alpha-olefins. Organoaluminium compounds are used as catalysts for alkene polymerization
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
to polyolefins
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
, for example the catalyst methylaluminoxane
Methylaluminoxane, commonly called MAO, is a mixture of organoaluminium compounds with the approximate formula (Al(CH3)O)''n''. It is usually encountered as a solution in (aromatic) solvents, commonly toluene but also xylene, cumene, or mesitylene ...
.
References
{{Authority control