nucleosome core particle on:
[Wikipedia]
[Google]
[Amazon]
 A nucleosome is the basic structural unit of DNA packaging in eukaryotes. The structure of a nucleosome consists of a segment of DNA wound around eight histone proteins and resembles thread wrapped around a spool. The nucleosome is the fundamental subunit of
A nucleosome is the basic structural unit of DNA packaging in eukaryotes. The structure of a nucleosome consists of a segment of DNA wound around eight histone proteins and resembles thread wrapped around a spool. The nucleosome is the fundamental subunit of
 Nucleosome core particles are observed when chromatin in interphase is treated to cause the chromatin to unfold partially. The resulting image, via an electron microscope, is "beads on a string". The string is the DNA, while each bead in the nucleosome is a core particle. The nucleosome core particle is composed of DNA and histone proteins.
Partial
Nucleosome core particles are observed when chromatin in interphase is treated to cause the chromatin to unfold partially. The resulting image, via an electron microscope, is "beads on a string". The string is the DNA, while each bead in the nucleosome is a core particle. The nucleosome core particle is composed of DNA and histone proteins.
Partial
 Since they were discovered in the mid-1960s, histone modifications have been predicted to affect transcription. The fact that most of the early post-translational modifications found were concentrated within the tail extensions that protrude from the nucleosome core lead to two main theories regarding the mechanism of histone modification. The first of the theories suggested that they may affect electrostatic interactions between the histone tails and DNA to "loosen" chromatin structure. Later it was proposed that combinations of these modifications may create binding epitopes with which to recruit other proteins. Recently, given that more modifications have been found in the structured regions of histones, it has been put forward that these modifications may affect histone-DNA and histone-histone interactions within the nucleosome core. Modifications (such as acetylation or phosphorylation) that lower the charge of the globular histone core are predicted to "loosen" core-DNA association; the strength of the effect depends on location of the modification within the core.
Some modifications have been shown to be correlated with gene silencing; others seem to be correlated with gene activation. Common modifications include acetylation, methylation, or
Since they were discovered in the mid-1960s, histone modifications have been predicted to affect transcription. The fact that most of the early post-translational modifications found were concentrated within the tail extensions that protrude from the nucleosome core lead to two main theories regarding the mechanism of histone modification. The first of the theories suggested that they may affect electrostatic interactions between the histone tails and DNA to "loosen" chromatin structure. Later it was proposed that combinations of these modifications may create binding epitopes with which to recruit other proteins. Recently, given that more modifications have been found in the structured regions of histones, it has been put forward that these modifications may affect histone-DNA and histone-histone interactions within the nucleosome core. Modifications (such as acetylation or phosphorylation) that lower the charge of the globular histone core are predicted to "loosen" core-DNA association; the strength of the effect depends on location of the modification within the core.
Some modifications have been shown to be correlated with gene silencing; others seem to be correlated with gene activation. Common modifications include acetylation, methylation, or
 Nucleosomes are the basic packing unit of DNA built from histone proteins around which DNA is coiled. They serve as a scaffold for formation of higher order chromatin structure as well as for a layer of regulatory control of gene expression. Nucleosomes are quickly assembled onto newly synthesized DNA behind the replication fork.
Nucleosomes are the basic packing unit of DNA built from histone proteins around which DNA is coiled. They serve as a scaffold for formation of higher order chromatin structure as well as for a layer of regulatory control of gene expression. Nucleosomes are quickly assembled onto newly synthesized DNA behind the replication fork.
File:Nucleosome core particle 1EQZ v.3.jpg
File:Nucleosome core particle 1EQZ v.4.jpg
File:Nucleosome core particle 1EQZ v.5.jpg
The crystal structure of the nucleosome core particle () - different views showing details of histone folding and organization. Histones , , , and are coloured.
MBInfo - What are nucleosomes
*
Dynamic Remodeling of Individual Nucleosomes Across a Eukaryotic Genome in Response to Transcriptional Perturbation
Nucleosome positioning data and tools online (annotated list, constantly updated)
HistoneDB 2.0 - Database of histones and variants
at
chromatin
Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in r ...
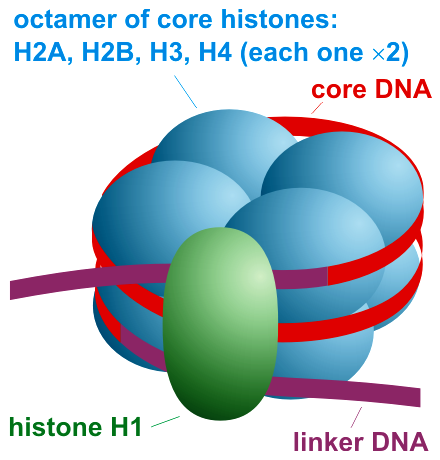
. Each nucleosome is composed of a little less than two turns of DNA wrapped around a set of eight proteins called histones, which are known as a histone octamer
A histone octamer is the eight-protein complex found at the center of a nucleosome core particle. It consists of two copies of each of the four core histone proteins ( H2A, H2B, H3, and H4). The octamer assembles when a tetramer, containing two ...
. Each histone octamer is composed of two copies each of the histone proteins H2A, H2B, H3, and H4.
DNA must be compacted into nucleosomes to fit within the cell nucleus. In addition to nucleosome wrapping, eukaryotic chromatin
Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in r ...
is further compacted by being folded into a series of more complex structures, eventually forming a chromosome
A chromosome is a long DNA molecule with part or all of the genetic material of an organism. In most chromosomes the very long thin DNA fibers are coated with packaging proteins; in eukaryotic cells the most important of these proteins are ...
. Each human cell contains about 30 million nucleosomes.
Nucleosomes are thought to carry epigenetically
In biology, epigenetics is the study of stable phenotypic changes (known as ''marks'') that do not involve alterations in the DNA sequence. The Greek prefix '' epi-'' ( "over, outside of, around") in ''epigenetics'' implies features that are "o ...
inherited information in the form of covalent modifications of their core histones
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn ar ...
. Nucleosome positions in the genome are not random, and it is important to know where each nucleosome is located because this determines the accessibility of the DNA to regulatory proteins.
Nucleosomes were first observed as particles in the electron microscope by Don and Ada Olins in 1974, and their existence and structure (as histone octamers surrounded by approximately 200 base pairs of DNA) were proposed by Roger Kornberg
Roger David Kornberg (born April 24, 1947) is an American biochemist and professor of structural biology at Stanford University School of Medicine. Kornberg was awarded the Nobel Prize in Chemistry in 2006 for his studies of the process by wh ...
. The role of the nucleosome as a regulator of transcription was demonstrated by Lorch et al. in vitro in 1987 and by Han and Grunstein and Clark-Adams et al. in vivo in 1988.
The nucleosome core particle consists of approximately 146 base pairs (bp) of DNAIn different crystals, values of 146 and 147 basepairs were observed wrapped in 1.67 left-handed superhelical turns around a histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn a ...
octamer, consisting of 2 copies each of the core histones H2A, H2B, H3, and H4. Core particles are connected by stretches of linker DNA, which can be up to about 80 bp long. Technically, a nucleosome is defined as the core particle plus one of these linker regions; however the word is often synonymous with the core particle. Genome-wide nucleosome positioning maps are now available for many model organisms including mouse liver and brain.
Linker histones such as H1 and its isoforms are involved in chromatin compaction and sit at the base of the nucleosome near the DNA entry and exit binding to the linker region of the DNA. Non-condensed nucleosomes without the linker histone resemble "beads on a string of DNA" under an electron microscope
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
.
In contrast to most eukaryotic cells, mature sperm cells largely use protamines
Protamines are small, arginine-rich, nuclear proteins that replace histones late in the haploid phase of spermatogenesis and are believed essential for sperm head condensation and DNA stabilization. They may allow for denser packaging of DNA in t ...
to package their genomic DNA, most likely to achieve an even higher packaging ratio. Histone equivalents and a simplified chromatin structure have also been found in Archaea, suggesting that eukaryotes are not the only organisms that use nucleosomes.
Structure
Structure of the core particle
Overview
Pioneering structural studies in the 1980s by Aaron Klug's group provided the first evidence that an octamer of histone proteins wraps DNA around itself in about 1.7 turns of a left-handed superhelix. In 1997 the first near atomic resolutioncrystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns ...
of the nucleosome was solved by the Richmond group, showing the most important details of the particle. The human alpha satellite palindromic DNA critical to achieving the 1997 nucleosome crystal structure was developed by the Bunick group at Oak Ridge National Laboratory in Tennessee. The structures of over 20 different nucleosome core particles have been solved to date, including those containing histone variants and histones from different species. The structure of the nucleosome core particle is remarkably conserved, and even a change of over 100 residues between frog and yeast histones results in electron density maps with an overall root mean square deviation
The root-mean-square deviation (RMSD) or root-mean-square error (RMSE) is a frequently used measure of the differences between values (sample or population values) predicted by a model or an estimator and the values observed. The RMSD represents ...
of only 1.6Å.
The nucleosome core particle (NCP)
The nucleosome core particle (shown in the figure) consists of about 146 base pair of DNA wrapped in 1.67 left-handed superhelical turns around thehistone octamer
A histone octamer is the eight-protein complex found at the center of a nucleosome core particle. It consists of two copies of each of the four core histone proteins ( H2A, H2B, H3, and H4). The octamer assembles when a tetramer, containing two ...
, consisting of 2 copies each of the core histones H2A, H2B, H3, and H4. Adjacent nucleosomes are joined by a stretch of free DNA termed linker DNA (which varies from 10 - 80 bp in length depending on species and tissue type).The whole structure generates a cylinder of diameter 11 nm and a height of 5.5 nm.


 Nucleosome core particles are observed when chromatin in interphase is treated to cause the chromatin to unfold partially. The resulting image, via an electron microscope, is "beads on a string". The string is the DNA, while each bead in the nucleosome is a core particle. The nucleosome core particle is composed of DNA and histone proteins.
Partial
Nucleosome core particles are observed when chromatin in interphase is treated to cause the chromatin to unfold partially. The resulting image, via an electron microscope, is "beads on a string". The string is the DNA, while each bead in the nucleosome is a core particle. The nucleosome core particle is composed of DNA and histone proteins.
Partial DNAse Deoxyribonuclease (DNase, for short) refers to a group of glycoprotein endonucleases which are enzymes that catalyze the hydrolytic cleavage of phosphodiester linkages in the DNA backbone, thus degrading DNA. The role of the DNase enzyme in cells ...
digestion of chromatin
Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in r ...
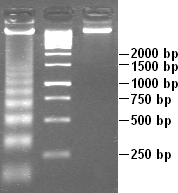
reveals its nucleosome structure. Because DNA portions of nucleosome core particles are less accessible for DNAse than linking sections, DNA gets digested into fragments of lengths equal to multiplicity of distance between nucleosomes (180, 360, 540 base pairs etc.). Hence a very characteristic pattern similar to a ladder is visible during gel electrophoresis of that DNA. Such digestion can occur also under natural conditions during apoptosis ("cell suicide" or programmed cell death), because autodestruction of DNA typically is its role.
=Protein interactions within the nucleosome
= The core histone proteins contains a characteristic structural motif termed the "histone fold", which consists of three alpha-helices (α1-3) separated by two loops (L1-2). In solution, the histones form H2A-H2B heterodimers and H3-H4 heterotetramers. Histones dimerise about their long α2 helices in an anti-parallel orientation, and, in the case of H3 and H4, two such dimers form a 4-helix bundle stabilised by extensive H3-H3' interaction. The H2A/H2B dimer binds onto the H3/H4 tetramer due to interactions between H4 and H2B, which include the formation of a hydrophobic cluster. The histone octamer is formed by a central H3/H4 tetramer sandwiched between two H2A/H2B dimers. Due to the highly basic charge of all four core histones, the histone octamer is stable only in the presence of DNA or very high salt concentrations.=Histone - DNA interactions
= The nucleosome contains over 120 direct protein-DNA interactions and several hundred water-mediated ones. Direct protein - DNA interactions are not spread evenly about the octamer surface but rather located at discrete sites. These are due to the formation of two types of DNA binding sites within the octamer; the α1α1 site, which uses the α1 helix from two adjacent histones, and the L1L2 site formed by the L1 and L2 loops. Salt links andhydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
between both side-chain basic and hydroxyl groups and main-chain amides with the DNA backbone phosphates form the bulk of interactions with the DNA. This is important, given that the ubiquitous distribution of nucleosomes along genomes requires it to be a non-sequence-specific DNA-binding factor. Although nucleosomes tend to prefer some DNA sequences over others, they are capable of binding practically to any sequence, which is thought to be due to the flexibility in the formation of these water-mediated interactions. In addition, non-polar interactions are made between protein side-chains and the deoxyribose groups, and an arginine side-chain intercalates into the DNA minor groove at all 14 sites where it faces the octamer surface.
The distribution and strength of DNA-binding sites about the octamer surface distorts the DNA within the nucleosome core. The DNA is non-uniformly bent and also contains twist defects. The twist of free B-form DNA in solution is 10.5 bp per turn. However, the overall twist of nucleosomal DNA is only 10.2 bp per turn, varying from a value of 9.4 to 10.9 bp per turn.
Histone tail domains
The histone tail extensions constitute up to 30% by mass of histones, but are not visible in the crystal structures of nucleosomes due to their high intrinsic flexibility, and have been thought to be largely unstructured. The N-terminal tails of histones H3 and H2B pass through a channel formed by the minor grooves of the two DNA strands, protruding from the DNA every 20 bp. The N-terminal tail of histone H4, on the other hand, has a region of highly basic amino acids (16-25), which, in the crystal structure, forms an interaction with the highly acidic surface region of a H2A-H2B dimer of another nucleosome, being potentially relevant for the higher-order structure of nucleosomes. This interaction is thought to occur under physiological conditions also, and suggests that acetylation of the H4 tail distorts the higher-order structure of chromatin.Higher order structure
The organization of the DNA that is achieved by the nucleosome cannot fully explain the packaging of DNA observed in the cell nucleus. Further compaction ofchromatin
Chromatin is a complex of DNA and protein found in eukaryotic cells. The primary function is to package long DNA molecules into more compact, denser structures. This prevents the strands from becoming tangled and also plays important roles in r ...
into the cell nucleus is necessary, but it is not yet well understood. The current understanding is that repeating nucleosomes with intervening "linker" DNA form a ''10-nm-fiber'', described as "beads on a string", and have a packing ratio of about five to ten. A chain of nucleosomes can be arranged in a ''30 nm fiber'', a compacted structure with a packing ratio of ~50 and whose formation is dependent on the presence of the H1 histone.
A crystal structure of a tetranucleosome has been presented and used to build up a proposed structure of the 30 nm fiber as a two-start helix.
There is still a certain amount of contention regarding this model, as it is incompatible with recent electron microscopy data. Beyond this, the structure of chromatin is poorly understood, but it is classically suggested that the 30 nm fiber is arranged into loops along a central protein scaffold to form transcriptionally active euchromatin
Euchromatin (also called "open chromatin") is a lightly packed form of chromatin ( DNA, RNA, and protein) that is enriched in genes, and is often (but not always) under active transcription. Euchromatin stands in contrast to heterochromatin, whi ...
. Further compaction leads to transcriptionally inactive heterochromatin.
Dynamics
Although the nucleosome is a very stable protein-DNA complex, it is not static and has been shown to undergo a number of different structural re-arrangements including nucleosome sliding and DNA site exposure. Depending on the context, nucleosomes can inhibit or facilitate transcription factor binding. Nucleosome positions are controlled by three major contributions: First, the intrinsic binding affinity of the histone octamer depends on the DNA sequence. Second, the nucleosome can be displaced or recruited by the competitive or cooperative binding of other protein factors. Third, the nucleosome may be actively translocated by ATP-dependent remodeling complexes.Nucleosome sliding
Work performed in the Bradbury laboratory showed that nucleosomes reconstituted onto the 5S DNA positioning sequence were able to reposition themselves translationally onto adjacent sequences when incubated thermally. Later work showed that this repositioning did not require disruption of the histone octamer but was consistent with nucleosomes being able to "slide" along the DNA ''in cis''. In 2008, it was further revealed thatCTCF
Transcriptional repressor CTCF also known as 11-zinc finger protein or CCCTC-binding factor is a transcription factor that in humans is encoded by the ''CTCF'' gene. CTCF is involved in many cellular processes, including transcriptional regulatio ...
binding sites act as nucleosome positioning anchors so that, when used to align various genomic signals, multiple flanking nucleosomes can be readily identified. Although nucleosomes are intrinsically mobile, eukaryotes have evolved a large family of ATP-dependent chromatin remodelling enzymes to alter chromatin structure, many of which do so via nucleosome sliding. In 2012, Beena Pillai's laboratory has demonstrated that nucleosome sliding is one of the possible mechanism for large scale tissue specific expression of genes. The work shows that the transcription start site for genes expressed in a particular tissue, are nucleosome depleted while, the same set of genes in other tissue where they are not expressed, are nucleosome bound.
DNA site exposure
Work from the Widom laboratory has shown that nucleosomal DNA is in equilibrium between a wrapped and unwrapped state. Measurements of these rates using time-resolved FRET revealed that DNA within the nucleosome remains fully wrapped for only 250 ms before it is unwrapped for 10-50 ms and then rapidly rewrapped. This implies that DNA does not need to be actively dissociated from the nucleosome but that there is a significant fraction of time during which it is fully accessible. Indeed, this can be extended to the observation that introducing a DNA-binding sequence within the nucleosome increases the accessibility of adjacent regions of DNA when bound. This propensity for DNA within the nucleosome to "breathe" has important functional consequences for all DNA-binding proteins that operate in a chromatin environment. In particular, the dynamic breathing of nucleosomes plays an important role in restricting the advancement ofRNA polymerase II
RNA polymerase II (RNAP II and Pol II) is a multiprotein complex that transcribes DNA into precursors of messenger RNA (mRNA) and most small nuclear RNA (snRNA) and microRNA. It is one of the three RNAP enzymes found in the nucleus of eukaryo ...
during transcription elongation.
Nucleosome free region
Promoters of active genes have nucleosome free regions (NFR). This allows for promoter DNA accessibility to various proteins, such as transcription factors. Nucleosome free region typically spans for 200 nucleotides in ''S. cerevisiae'' Well-positioned nucleosomes form boundaries of NFR. These nucleosomes are called +1-nucleosome and −1-nucleosome and are located at canonical distances downstream and upstream, respectively, from transcription start site. +1-nucleosome and several downstream nucleosomes also tend to incorporate H2A.Z histone variant.Modulating nucleosome structure
Eukaryotic genomes are ubiquitously associated into chromatin; however, cells must spatially and temporally regulate specific loci independently of bulk chromatin. In order to achieve the high level of control required to co-ordinate nuclear processes such as DNA replication, repair, and transcription, cells have developed a variety of means to locally and specifically modulate chromatin structure and function. This can involve covalent modification of histones, the incorporation of histone variants, and non-covalent remodelling by ATP-dependent remodeling enzymes.Histone post-translational modifications
ubiquitination
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
of lysine; methylation of arginine; and phosphorylation of serine. The information stored in this way is considered epigenetic, since it is not encoded in the DNA but is still inherited to daughter cells. The maintenance of a repressed or activated status of a gene is often necessary for cellular differentiation.
Histone variants
Although histones are remarkably conserved throughout evolution, several variant forms have been identified. This diversification of histone function is restricted to H2A and H3, with H2B and H4 being mostly invariant. H2A can be replaced by H2AZ (which leads to reduced nucleosome stability) orH2AX
H2A histone family member X (usually abbreviated as H2AX) is a type of histone protein from the H2A family encoded by the ''H2AFX'' gene. An important phosphorylated form is γH2AX (S139), which forms when double-strand breaks appear.
In humans ...
(which is associated with DNA repair and T cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell r ...
differentiation), whereas the inactive X chromosomes in mammals are enriched in macroH2A. H3 can be replaced by H3.3 (which correlates with activate genes and regulatory elements) and in centromere
The centromere links a pair of sister chromatids together during cell division. This constricted region of chromosome connects the sister chromatids, creating a short arm (p) and a long arm (q) on the chromatids. During mitosis, spindle fibers ...
s H3 is replaced by CENPA.
ATP-dependent nucleosome remodeling
A number of distinct reactions are associated with the termATP-dependent chromatin remodeling
Chromatin remodeling is the dynamic modification of chromatin architecture to allow access of condensed genomic DNA to the regulatory Transcription (genetics), transcription machinery proteins, and thereby control gene expression. Such remodeling i ...
. Remodeling enzymes have been shown to slide nucleosomes along DNA, disrupt histone-DNA contacts to the extent of destabilizing the H2A/H2B dimer and to generate negative superhelical torsion in DNA and chromatin. Recently, the Swr1 remodeling enzyme has been shown to introduce the variant histone H2A.Z into nucleosomes. At present, it is not clear if all of these represent distinct reactions or merely alternative outcomes of a common mechanism. What is shared between all, and indeed the hallmark of ATP-dependent chromatin remodeling, is that they all result in altered DNA accessibility.
Studies looking at gene activation ''in vivo'' and, more astonishingly, remodeling ''in vitro'' have revealed that chromatin remodeling events and transcription-factor binding are cyclical and periodic in nature. While the consequences of this for the reaction mechanism of chromatin remodeling are not known, the dynamic nature of the system may allow it to respond faster to external stimuli. A recent study indicates that nucleosome positions change significantly during mouse embryonic stem cell development, and these changes are related to binding of developmental transcription factors.
Dynamic nucleosome remodelling across the Yeast genome
Studies in 2007 have catalogued nucleosome positions in yeast and shown that nucleosomes are depleted in promoter regions and origins of replication. About 80% of the yeast genome appears to be covered by nucleosomes and the pattern of nucleosome positioning clearly relates to DNA regions that regulatetranscription
Transcription refers to the process of converting sounds (voice, music etc.) into letters or musical notes, or producing a copy of something in another medium, including:
Genetics
* Transcription (biology), the copying of DNA into RNA, the fir ...
, regions that are transcribed and regions that initiate DNA replication. Most recently, a new study examined ''dynamic changes'' in nucleosome repositioning during a global transcriptional reprogramming event to elucidate the effects on nucleosome displacement during genome-wide transcriptional changes in yeast (''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have b ...
''). The results suggested that nucleosomes that were localized to promoter regions are displaced in response to stress (like heat shock
The heat shock response (HSR) is a cell stress response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a normal ...
). In addition, the removal of nucleosomes usually corresponded to transcriptional activation and the replacement of nucleosomes usually corresponded to transcriptional repression, presumably because transcription factor
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The f ...
binding sites became more or less accessible, respectively. In general, only one or two nucleosomes were repositioned at the promoter to effect these transcriptional changes. However, even in chromosomal regions that were not associated with transcriptional changes, nucleosome repositioning was observed, suggesting that the covering and uncovering of transcriptional DNA does not necessarily produce a transcriptional event. After transcription, the rDNA region has to protected from any damage, it suggested HMGB proteins play a major role in protecting the nucleosome free region.
DNA Twist Defects
DNA twist defects are when the addition of one or a few base pairs from one DNA segment are transferred to the next segment resulting in a change of the DNA twist. This will not only change the twist of the DNA but it will also change the length. This twist defect eventually moves around the nucleosome through the transferring of the base pair, this means DNA twists can cause nucleosome sliding. Nucleosome crystal structures have shown that superhelix location 2 and 5 on the nucleosome are commonly found to be where DNA twist defects occur as these are common remodeler binding sites. There are a variety of chromatin remodelers but all share the existence of an ATPase motor which facilitates chromatin sliding on DNA through the binding and hydrolysis of ATP. ATPase has an open and closed state. When the ATPase motor is changing from open and closed states, the DNA duplex changes geometry and exhibits base pair tilting. The initiation of the twist defects via the ATPase motor causes tension to accumulate around the remodeler site. The tension is released when the sliding of DNA has been completed throughout the nucleosome via the spread of two twist defects (one on each strand) in opposite directions.Nucleosome assembly ''in vitro''
Nucleosomes can be assembled ''in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called " test-tube experiments", these studies in biology ...
'' by either using purified native or recombinant histones. One standard technique of loading the DNA around the histones involves the use of salt dialysis. A reaction consisting of the histone octamers and a naked DNA template can be incubated together at a salt concentration of 2 M. By steadily decreasing the salt concentration, the DNA will equilibrate to a position where it is wrapped around the histone octamers, forming nucleosomes. In appropriate conditions, this reconstitution process allows for the nucleosome positioning affinity of a given sequence to be mapped experimentally.
Disulfide crosslinked nucleosome core particles
A recent advance in the production of nucleosome core particles with enhanced stability involves site-specificdisulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
crosslinks. Two different crosslinks can be introduced into the nucleosome core particle. A first one crosslinks the two copies of H2A via an introduced cysteine (N38C) resulting in histone octamer
A histone octamer is the eight-protein complex found at the center of a nucleosome core particle. It consists of two copies of each of the four core histone proteins ( H2A, H2B, H3, and H4). The octamer assembles when a tetramer, containing two ...
which is stable against H2A/H2B dimer loss during nucleosome reconstitution. A second crosslink can be introduced between the H3 N-terminal histone tail and the nucleosome DNA ends via an incorporated convertible nucleotide. The DNA-histone octamer crosslink stabilizes the nucleosome core particle against DNA dissociation at very low particle concentrations and at elevated salt concentrations.
Nucleosome assembly '' in vivo ''
H3 and H4
Histones H3 and H4 from disassembled old nucleosomes are kept in the vicinity and randomly distributed on the newly synthesized DNA. They are assembled by the chromatin assembly factor-1 (CAF-1) complex, which consists of three subunits (p150, p60, and p48). Newly synthesized H3 and H4 are assembled by the replication coupling assembly factor (RCAF). RCAF contains the subunit Asf1, which binds to newly synthesized H3 and H4 proteins. The old H3 and H4 proteins retain their chemical modifications which contributes to the passing down of the epigenetic signature. The newly synthesized H3 and H4 proteins are gradually acetylated at different lysine residues as part of the chromatin maturation process. It is also thought that the old H3 and H4 proteins in the new nucleosomes recruit histone modifying enzymes that mark the new histones, contributing to epigenetic memory.H2A and H2B
In contrast to old H3 and H4, the old H2A and H2B histone proteins are released and degraded; therefore, newly assembled H2A and H2B proteins are incorporated into new nucleosomes. H2A and H2B are assembled into dimers which are then loaded onto nucleosomes by the nucleosome assembly protein-1 (NAP-1) which also assists with nucleosome sliding. The nucleosomes are also spaced by ATP-dependent nucleosome-remodeling complexes containing enzymes such as Isw1 Ino80, and Chd1, and subsequently assembled into higher order structure.Gallery
See also
* ChromomereReferences
External links
MBInfo - What are nucleosomes
*
Dynamic Remodeling of Individual Nucleosomes Across a Eukaryotic Genome in Response to Transcriptional Perturbation
Nucleosome positioning data and tools online (annotated list, constantly updated)
HistoneDB 2.0 - Database of histones and variants
at
NCBI
The National Center for Biotechnology Information (NCBI) is part of the United States National Library of Medicine (NLM), a branch of the National Institutes of Health (NIH). It is approved and funded by the government of the United States. The ...
{{Use dmy dates, date=April 2017
Molecular biology
Epigenetics
Nuclear organization