Nickel on:
[Wikipedia]
[Google]
[Amazon]
Nickel is a
 Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are ferromagnetic at or near room temperature; the others are iron, cobalt and
Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are ferromagnetic at or near room temperature; the others are iron, cobalt and
To read the nickel atom levels, type "Ni I" in the Spectrum box and click on Retrieve data. However, each of these two configurations splits into several energy levels due to fine structure, and the two sets of energy levels overlap. The average energy of states with r3d 4s is actually lower than the average energy of states with r3d 4s. Therefore, the research literature on atomic calculations quotes the ground state configuration as r3d 4s.
 On Earth, nickel occurs most often in combination with sulfur and iron in
On Earth, nickel occurs most often in combination with sulfur and iron in
 Nickel tetracarbonyl ), discovered by Ludwig Mond, is a volatile, highly toxic liquid at room temperature. On heating, the complex decomposes back to nickel and carbon monoxide:
:
This behavior is exploited in the Mond process for purifying nickel, as described above. The related nickel(0) complex bis(cyclooctadiene)nickel(0) is a useful catalyst in organonickel chemistry because the cyclooctadiene (or ''cod'') ligands are easily displaced.
Nickel tetracarbonyl ), discovered by Ludwig Mond, is a volatile, highly toxic liquid at room temperature. On heating, the complex decomposes back to nickel and carbon monoxide:
:
This behavior is exploited in the Mond process for purifying nickel, as described above. The related nickel(0) complex bis(cyclooctadiene)nickel(0) is a useful catalyst in organonickel chemistry because the cyclooctadiene (or ''cod'') ligands are easily displaced.
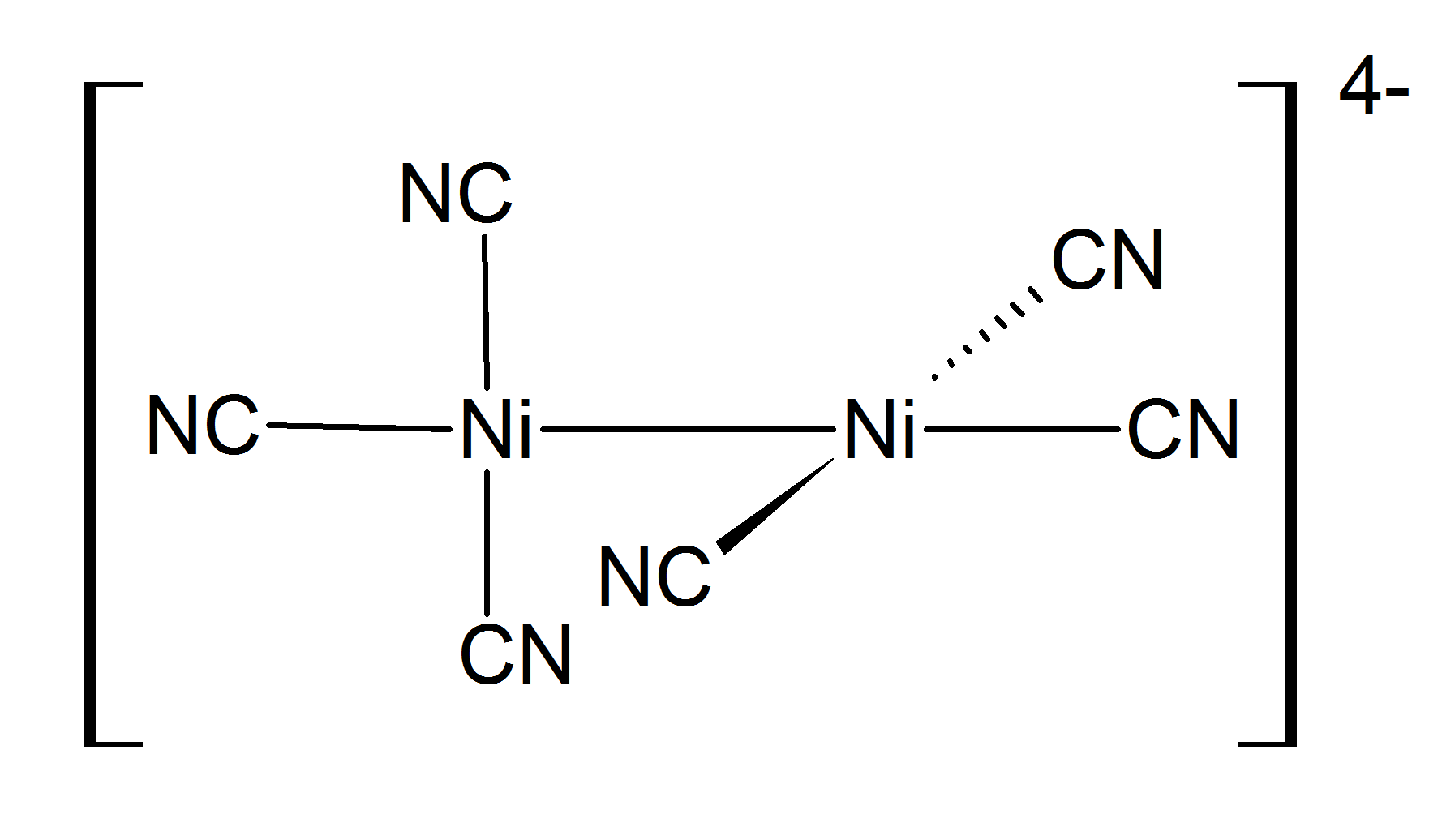 Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red
Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red

 Nickel(II) forms compounds with all common anions, including sulfide,
Nickel(II) forms compounds with all common anions, including sulfide,
 Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R = ethyl,
Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R = ethyl,
 In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral
In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral
 Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
 In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the
In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the

 An estimated 2.7 million tonnes (t) of nickel per year are mined worldwide; Indonesia (1,000,000 t), the
An estimated 2.7 million tonnes (t) of nickel per year are mined worldwide; Indonesia (1,000,000 t), the
 Nickel is obtained through extractive metallurgy: it is extracted from ore by conventional roasting and reduction processes that yield metal of greater than 75% purity. In many stainless steel applications, 75% pure nickel can be used without further purification, depending on impurities.
Traditionally, most sulfide ores are processed using
Nickel is obtained through extractive metallurgy: it is extracted from ore by conventional roasting and reduction processes that yield metal of greater than 75% purity. In many stainless steel applications, 75% pure nickel can be used without further purification, depending on impurities.
Traditionally, most sulfide ores are processed using 
 The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form
The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form
 Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9% electroplating, 7% alloy steel, 3% foundries, and 4% other (including batteries).
Nickel is used in many recognizable industrial and consumer products, including stainless steel,
Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9% electroplating, 7% alloy steel, 3% foundries, and 4% other (including batteries).
Nickel is used in many recognizable industrial and consumer products, including stainless steel,  Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper stainless steel (i.e., iron) alloys, except in the United States and Canada.
Nickel is an excellent alloying agent for certain precious metals and is used in the
Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper stainless steel (i.e., iron) alloys, except in the United States and Canada.
Nickel is an excellent alloying agent for certain precious metals and is used in the
"Nickel and nickel compounds"
in ''IARC Monogr Eval Carcinog Risks Hum''. Volume 100C. pp. 169–218..Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 J L 353, 31.12.2008, p. 1br>Annex VI
. Accessed July 13, 2017.Globally Harmonised System of Classification and Labelling of Chemicals (GHS)
, 5th ed., United Nations, New York and Geneva, 2013..National Toxicology Program. (2016)
, 14th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.. based on increased respiratory cancer risks observed in epidemiological studies of sulfidic ore refinery workers. This is supported by the positive results of the NTP bioassays with Ni sub-sulfide and Ni oxide in rats and mice. The human and animal data consistently indicate a lack of carcinogenicity via the oral route of exposure and limit the carcinogenicity of nickel compounds to respiratory tumours after inhalation. Nickel metal is classified as a suspect carcinogen; there is consistency between the absence of increased respiratory cancer risks in workers predominantly exposed to metallic nickel and the lack of respiratory tumours in a rat lifetime inhalation carcinogenicity study with nickel metal powder. In the rodent inhalation studies with various nickel compounds and nickel metal, increased lung inflammations with and without bronchial lymph node hyperplasia or fibrosis were observed. In rat studies, oral ingestion of water-soluble nickel salts can trigger perinatal mortality in pregnant animals. Whether these effects are relevant to humans is unclear as epidemiological studies of highly exposed female workers have not shown adverse developmental toxicity effects. People can be exposed to nickel in the workplace by inhalation, ingestion, and contact with skin or eye. The Occupational Safety and Health Administration (OSHA) has set the legal limit ( permissible exposure limit) for the workplace at 1 mg/m per 8-hour workday, excluding nickel carbonyl. The
. American Academy of Dermatology(August 22, 2015) Reports show that both the nickel-induced activation of hypoxia-inducible factor (HIF-1) and the up-regulation of hypoxia-inducible genes are caused by depletion of intracellular ascorbate. The addition of ascorbate to the culture medium increased the intracellular ascorbate level and reversed both the metal-induced stabilization of HIF-1- and HIF-1α-dependent gene expression.
Nickel
at ''
CDC – Nickel – NIOSH Workplace Safety and Health Topic
An occupational hygiene assessment of dermal nickel exposures in primary production industries
by GW Hughson.
An occupational hygiene assessment of dermal nickel exposures in primary production and primary user industries. Phase 2 Report
by GW Hughson.
"The metal that brought you cheap flights"
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union ...
because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere.
Meteoric nickel is found in combination with iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores.
Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an element in 1751 by Axel Fredrik Cronstedt
Baron Axel Fredrik Cronstedt (''/kroonstet/'' 23 December 1722 – 19 August 1765) was a Swedish mineralogist and chemist who discovered the element nickel in 1751 as a mining expert with the Bureau of Mines.
Cronstedt is considered a founder ...
, who initially mistook the ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
for a copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
, in the cobalt mines of Los, Hälsingland, Sweden. The element's name comes from a mischievous sprite of German miner mythology, Nickel (similar to Old Nick), who personified the fact that copper-nickel ores resisted refinement into copper. An economically important source of nickel is the iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
ore limonite, which is often 1–2% nickel. Other important nickel ore minerals include pentlandite
Pentlandite is an iron–nickel sulfide with the chemical formula . Pentlandite has a narrow variation range in Ni:Fe but it is usually described as having a Ni:Fe of 1:1. It also contains minor cobalt, usually at low levels as a fraction of wei ...
and a mix of Ni-rich natural silicates known as garnierite
Garnierite is a general name for a green nickel ore which is found in pockets and veins within weathered and serpentinized ultramafic rocks. It forms by lateritic weathering of ultramafic rocks and occurs in many nickel laterite deposits in the ...
. Major production sites include the Sudbury region, Canada
Canada is a country in North America. Its ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, covering over , making it the world's second-largest country by tot ...
(which is thought to be of meteoric origin), New Caledonia in the Pacific
The Pacific Ocean is the largest and deepest of Earth's five oceanic divisions. It extends from the Arctic Ocean in the north to the Southern Ocean (or, depending on definition, to Antarctica) in the south, and is bounded by the contine ...
, and Norilsk
Norilsk ( rus, Нори́льск, p=nɐˈrʲilʲsk, ''Norílʹsk'') is a closed city in Krasnoyarsk Krai, Russia, located south of the western Taymyr Peninsula, around 90 km east of the Yenisey River and 1,500 km north of Krasnoyarsk. Norilsk ...
, Russia
Russia (, , ), or the Russian Federation, is a transcontinental country spanning Eastern Europe and Northern Asia. It is the largest country in the world, with its internationally recognised territory covering , and encompassing one-eig ...
.
Nickel is one of four elements (the others are iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
, cobalt, and gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
) that are ferromagnetic at about room temperature. Alnico
Alnico is a family of iron alloys which in addition to iron are composed primarily of aluminium (Al), nickel (Ni), and cobalt (Co), hence the acronym ''al-ni-co''. They also include copper, and sometimes titanium. Alnico alloys are ferromagnetic, ...
permanent magnets
A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel ...
based partly on nickel are of intermediate strength between iron-based permanent magnets and rare-earth magnets. The metal is used chiefly in alloys and corrosion-resistant plating. About 68% of world production is used in stainless steel. A further 10% is used for nickel-based and copper-based alloys, 9% for plating, 7% for alloy steels, 3% in foundries, and 4% in other applications such as in rechargeable batteries, including those in electric vehicles (EVs). Nickel is widely used in coins
A coin is a small, flat (usually depending on the country or value), round piece of metal or plastic used primarily as a medium of exchange or legal tender. They are standardized in weight, and produced in large quantities at a mint in order t ...
, though nickel-plated objects sometimes provoke nickel allergy
Nickel allergy or nickel allergic contact dermatitis (Ni-ACD) is a form of allergic contact dermatitis (ACD) caused by exposure to the chemical element nickel. It typically causes a rash that is red and itchy and that may be bumpy or scaly. The ...
. As a compound, nickel has a number of niche chemical manufacturing uses, such as a catalyst for hydrogenation, cathodes for rechargeable batteries, pigments and metal surface treatments. Nickel is an essential nutrient for some microorganisms and plants that have enzymes with nickel as an active site.
Properties
Atomic and physical properties
 Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are ferromagnetic at or near room temperature; the others are iron, cobalt and
Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are ferromagnetic at or near room temperature; the others are iron, cobalt and gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen ...
. Its Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
is , meaning that bulk nickel is non-magnetic above this temperature. The unit cell of nickel is a face-centered cube with the lattice parameter of 0.352 nm, giving an atomic radius of 0.124 nm. This crystal structure is stable to pressures of at least 70 GPa. Nickel is hard, malleable and ductile
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
, and has a relatively high electrical
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described ...
and thermal conductivity for transition metals. The high compressive strength of 34 GPa, predicted for ideal crystals, is never obtained in the real bulk material due to formation and movement of dislocations. However, it has been reached in Ni nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
s.
Electron configuration dispute
Nickel has two atomic electron configurations, r3d 4s and r3d 4s, which are very close in energy; rdenotes the completeargon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
core structure. There is some disagreement on which configuration has the lower energy. Chemistry textbooks quote nickel's electron configuration as r4s 3d, also written r3d 4s. This configuration agrees with the Madelung energy ordering rule, which predicts that 4s is filled before 3d. It is supported by the experimental fact that the lowest energy state of the nickel atom is a 3d 4s energy level, specifically the 3d(F) 4s F, ''J'' = 4 level.NIST Atomic Spectrum DatabaseTo read the nickel atom levels, type "Ni I" in the Spectrum box and click on Retrieve data. However, each of these two configurations splits into several energy levels due to fine structure, and the two sets of energy levels overlap. The average energy of states with r3d 4s is actually lower than the average energy of states with r3d 4s. Therefore, the research literature on atomic calculations quotes the ground state configuration as r3d 4s.
Isotopes
The isotopes of nickel range inatomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
from 48 u () to 78 u ().
Natural nickel is composed of five stable isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
s, , , , and , of which is the most abundant (68.077% natural abundance).
Nickel-62
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons.
It is a stable isotope, with the highest binding energy per nucleon of any known nuclide (8.7945 MeV). It is often stated that 56Fe is the "most stable nucleus", but only beca ...
has the highest binding energy per nucleon of any nuclide: 8.7946 MeV/nucleon. Its binding energy is greater than both and , more abundant nuclides often incorrectly cited as having the highest binding energy. Though this would seem to predict nickel as the most abundant heavy element in the universe, the high rate of photodisintegration
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The ...
of nickel in stellar interiors causes iron to be by far the most abundant.
Nickel-60 is the daughter product of the extinct radionuclide
An extinct radionuclide is a radionuclide that was formed by nucleosynthesis before the formation of the Solar System, about 4.6 billion years ago, but has since decayed to virtually zero abundance and is no longer detectable as a primordial nuc ...
(half-life 2.6 million years). Due to the long half-life of , its persistence in materials in the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
may generate observable variations in the isotopic composition of . Therefore, the abundance of in extraterrestrial material may give insight into the origin of the Solar System and its early history.
At least 26 nickel radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s have been characterized; the most stable are with half-life 76,000 years, (100 years), and (6 days). All other radioisotopes have half-lives less than 60 hours and most these have half-lives less than 30 seconds. This element also has one meta state
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ...
.
Radioactive nickel-56 is produced by the silicon burning process and later set free in large amounts in type Ia supernovae. The shape of the light curve
In astronomy, a light curve is a graph of light intensity of a celestial object or region as a function of time, typically with the magnitude of light received on the y axis and with time on the x axis. The light is usually in a particular frequ ...
of these supernovae at intermediate to late-times corresponds to the decay via electron capture of to cobalt-56 and ultimately to iron-56. Nickel-59 is a long-lived cosmogenic
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an ''in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom ...
radionuclide; half-life 76,000 years. has found many applications in isotope geology. has been used to date the terrestrial age of meteorites and to determine abundances of extraterrestrial dust in ice and sediment
Sediment is a naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of wind, water, or ice or by the force of gravity acting on the particles. For example, sa ...
. The half-life of nickel-78 was recently measured at 110 milliseconds, and is believed an important isotope in supernova nucleosynthesis of elements heavier than iron. Ni, discovered in 1999, is the most proton-rich heavy element isotope known. With 28 protons and 20 neutrons, Ni is " doubly magic", as is Ni with 28 protons and 50 neutrons. Both are therefore unusually stable for nuclei with so large a proton–neutron imbalance.
Nickel-63 is a contaminant found in the support structure of nuclear reactors. It is produced through neutron capture by nickel-62. Small amounts have also been found near nuclear weapon test sites in the South Pacific.
Occurrence
 On Earth, nickel occurs most often in combination with sulfur and iron in
On Earth, nickel occurs most often in combination with sulfur and iron in pentlandite
Pentlandite is an iron–nickel sulfide with the chemical formula . Pentlandite has a narrow variation range in Ni:Fe but it is usually described as having a Ni:Fe of 1:1. It also contains minor cobalt, usually at low levels as a fraction of wei ...
, with sulfur in millerite
Millerite is a nickel sulfide mineral, Ni S. It is brassy in colour and has an acicular habit, often forming radiating masses and furry aggregates. It can be distinguished from pentlandite by crystal habit, its duller colour, and general la ...
, with arsenic in the mineral nickeline
Nickeline or niccolite is a mineral consisting primarily of nickel arsenide (NiAs). The naturally-occurring mineral contains roughly 43.9% nickel and 56.1% arsenic by mass, but composition of the mineral may vary slightly.
Small quantities of ...
, and with arsenic and sulfur in nickel galena. Nickel is commonly found in iron meteorites as the alloys kamacite and taenite. Nickel in meteorites was first detected in 1799 by Joseph-Louis Proust, a French chemist who then worked in Spain. Proust analyzed samples of the meteorite from Campo del Cielo (Argentina), which had been obtained in 1783 by Miguel Rubín de Celis, discovering the presence in them of nickel (about 10%) along with iron.
The bulk of nickel is mined from two types of ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
deposits. The first is laterite, where the principal ore mineral mixtures are nickeliferous limonite, (Fe,Ni)O(OH), and garnierite
Garnierite is a general name for a green nickel ore which is found in pockets and veins within weathered and serpentinized ultramafic rocks. It forms by lateritic weathering of ultramafic rocks and occurs in many nickel laterite deposits in the ...
(a mixture of various hydrous nickel and nickel-rich silicates). The second is magmatic sulfide deposits, where the principal ore mineral is pentlandite
Pentlandite is an iron–nickel sulfide with the chemical formula . Pentlandite has a narrow variation range in Ni:Fe but it is usually described as having a Ni:Fe of 1:1. It also contains minor cobalt, usually at low levels as a fraction of wei ...
: .
Indonesia and Australia have the biggest estimated reserves, at 43.6% of world total.
Identified land-based resources throughout the world averaging 1% nickel or greater comprise at least 130 million tons of nickel (about the double of known reserves). About 60% is in laterites and 40% in sulfide deposits.
On geophysical
Geophysics () is a subject of natural science concerned with the physical processes and physical properties of the Earth and its surrounding space environment, and the use of quantitative methods for their analysis. The term ''geophysics'' some ...
evidence, most of the nickel on Earth is believed to be in Earth's outer and inner core
Earth's inner core is the innermost geologic layer of planet Earth. It is primarily a solid ball with a radius of about , which is about 20% of Earth's radius or 70% of the Moon's radius.
There are no samples of Earth's core accessible for d ...
s. Kamacite and taenite are naturally occurring alloys of iron and nickel. For kamacite, the alloy is usually in the proportion of 90:10 to 95:5, though impurities (such as cobalt or carbon) may be present. Taenite is 20% to 65% nickel. Kamacite and taenite are also found in nickel iron meteorites.
Compounds
The most common oxidation state of nickel is +2, but compounds of , , and are well known, and the exotic oxidation states and have been produced and studied.Nickel(0)
 Nickel tetracarbonyl ), discovered by Ludwig Mond, is a volatile, highly toxic liquid at room temperature. On heating, the complex decomposes back to nickel and carbon monoxide:
:
This behavior is exploited in the Mond process for purifying nickel, as described above. The related nickel(0) complex bis(cyclooctadiene)nickel(0) is a useful catalyst in organonickel chemistry because the cyclooctadiene (or ''cod'') ligands are easily displaced.
Nickel tetracarbonyl ), discovered by Ludwig Mond, is a volatile, highly toxic liquid at room temperature. On heating, the complex decomposes back to nickel and carbon monoxide:
:
This behavior is exploited in the Mond process for purifying nickel, as described above. The related nickel(0) complex bis(cyclooctadiene)nickel(0) is a useful catalyst in organonickel chemistry because the cyclooctadiene (or ''cod'') ligands are easily displaced.
Nickel(I)
 Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red
Nickel(I) complexes are uncommon, but one example is the tetrahedral complex . Many nickel(I) complexes have Ni–Ni bonding, such as the dark red diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
prepared by reduction of with sodium amalgam
Sodium amalgam, commonly denoted Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. Sodium am ...
. This compound is oxidized in water, liberating .
It is thought that the nickel(I) oxidation state is important to nickel-containing enzymes, such as iFehydrogenase, which catalyzes the reversible reduction of protons to .
Nickel(II)

 Nickel(II) forms compounds with all common anions, including sulfide,
Nickel(II) forms compounds with all common anions, including sulfide, sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
, carbonate, hydroxide, carboxylates, and halides. Nickel(II) sulfate is produced in large amounts by dissolving nickel metal or oxides in sulfuric acid, forming both a hexa- and heptahydrateLascelles, Keith; Morgan, Lindsay G.; Nicholls, David and Beyersmann, Detmar (2019) "Nickel Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry''. Wiley-VCH, Weinheim. useful for electroplating nickel. Common salts of nickel, such as chloride, nitrate, and sulfate, dissolve in water to give green solutions of the metal aquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
.
The four halides form nickel compounds, which are solids with molecules with octahedral Ni centres. Nickel(II) chloride is most common, and its behavior is illustrative of the other halides. Nickel(II) chloride is made by dissolving nickel or its oxide in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
. It is usually found as the green hexahydrate, whose formula is usually written . When dissolved in water, this salt forms the metal aquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
. Dehydration of gives yellow anhydrous .
Some tetracoordinate nickel(II) complexes, e.g. bis(triphenylphosphine)nickel chloride, exist both in tetrahedral and square planar geometries. The tetrahedral complexes are paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
; the square planar complexes are diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
. In having properties of magnetic equilibrium and formation of octahedral complexes, they contrast with the divalent complexes of the heavier group 10 metals, palladium(II) and platinum(II), which form only square-planar geometry.
Nickelocene
Nickelocene is the organonickel compound with the formula Ni( ''η''5-C5H5)2. Also known as bis(cyclopentadienyl)nickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it does not yet have any known prac ...
is known; it has an electron count of 20, making it relatively unstable.
Nickel(III) and (IV)
 Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R = ethyl,
Many Ni(III) compounds are known. The first such compounds are , where X = Cl, Br, I and R = ethyl, propyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often ...
, butyl
In organic chemistry, butyl is a four- carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, gi ...
. Further, Ni(III) forms simple salts with fluoride or oxide ions. Ni(III) can be stabilized by σ-donor ligands such as thiols and organophosphines.
Ni(III) occurs in nickel oxide hydroxide
Nickel oxide hydroxide is the inorganic compound with the chemical formula NiO(OH). It is a black solid that is insoluble in all solvents but attacked by base and acid. It is a component of the nickel-metal hydride battery and of the nickel–iro ...
, which is used as the cathode in many rechargeable batteries
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or prima ...
, including nickel-cadmium, nickel-iron, nickel hydrogen, and nickel-metal hydride, and used by certain manufacturers in Li-ion batteries.
Ni(IV) occurs in the mixed oxide . Ni(IV) remains a rare oxidation state and very few compounds are known.
History
Because nickel ores are easily mistaken for ores of silver and copper, understanding of this metal and its use, is relatively recent. But unintentional use of nickel is ancient, and can be traced back as far as 3500 BCE. Bronzes from what is now Syria have been found to contain as much as 2% nickel. Some ancient Chinese manuscripts suggest that "white copper" ( cupronickel, known as ''baitong'') was used there in 1700-1400 BCE. This Paktong white copper was exported to Britain as early as the 17th century, but the nickel content of this alloy was not discovered until 1822. Coins of nickel-copper alloy were minted by Bactrian kingsAgathocles Agathocles ( Greek: ) is a Greek name, the most famous of which is Agathocles of Syracuse, the tyrant of Syracuse. The name is derived from , ''agathos'', i.e. "good" and , ''kleos'', i.e. "glory".
Other personalities named Agathocles:
*Agathocles ...
, Euthydemus II, and Pantaleon
Pantaleon, also known as Panteleimon, (Greek: ) was a Greek king who reigned some time between 190–180 BC in Bactria and India. He was a younger contemporary or successor of the Greco-Bactrian king Demetrius, and is sometimes believed to ha ...
in the 2nd century BCE, possibly out of the Chinese cupronickel.
 In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral
In medieval Germany, a metallic yellow mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. But when miners were unable to get any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to '' Old Nick''), for besetting the copper. They called this ore ''Kupfernickel'' from German ''Kupfer'' 'copper'. This ore is now known as the mineral nickeline
Nickeline or niccolite is a mineral consisting primarily of nickel arsenide (NiAs). The naturally-occurring mineral contains roughly 43.9% nickel and 56.1% arsenic by mass, but composition of the mineral may vary slightly.
Small quantities of ...
(formerly ''niccolite''), a nickel arsenide
In chemistry, an arsenide is a compound of arsenic with a less electronegative element or elements. Many metals form binary compounds containing arsenic, and these are called arsenides. They exist with many stoichiometries, and in this respect a ...
. In 1751, Baron Axel Fredrik Cronstedt
Baron Axel Fredrik Cronstedt (''/kroonstet/'' 23 December 1722 – 19 August 1765) was a Swedish mineralogist and chemist who discovered the element nickel in 1751 as a mining expert with the Bureau of Mines.
Cronstedt is considered a founder ...
tried to extract copper from kupfernickel at a cobalt mine in the village of Los, Sweden, and instead produced a white metal that he named ''nickel'' after the spirit that had given its name to the mineral. In modern German, Kupfernickel or Kupfer-Nickel designates the alloy cupronickel.
Originally, the only source for nickel was the rare Kupfernickel. Beginning in 1824, nickel was obtained as a byproduct of cobalt blue production. The first large-scale smelting of nickel began in Norway in 1848 from nickel-rich pyrrhotite
Pyrrhotite is an iron sulfide mineral with the formula Fe(1-x)S (x = 0 to 0.2). It is a nonstoichiometric variant of FeS, the mineral known as troilite.
Pyrrhotite is also called magnetic pyrite, because the color is similar to pyrite and it i ...
. The introduction of nickel in steel production in 1889 increased the demand for nickel; the nickel deposits of New Caledonia, discovered in 1865, provided most of the world's supply between 1875 and 1915. The discovery of the large deposits in the Sudbury Basin, Canada in 1883, in Norilsk
Norilsk ( rus, Нори́льск, p=nɐˈrʲilʲsk, ''Norílʹsk'') is a closed city in Krasnoyarsk Krai, Russia, located south of the western Taymyr Peninsula, around 90 km east of the Yenisey River and 1,500 km north of Krasnoyarsk. Norilsk ...
-Talnakh, Russia in 1920, and in the Merensky Reef
The Merensky Reef is a layer of igneous rock in the Bushveld Igneous Complex (BIC) in the North West, Limpopo, Gauteng and Mpumalanga provinces of South Africa which together with an underlying layer, the Upper Group 2 Reef (UG2), contains most o ...
, South Africa in 1924, made large-scale nickel production possible.
Coinage
 Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
Aside from the aforementioned Bactrian coins, nickel was not a component of coins until the mid-19th century.
Canada
99.9% nickel five-cent coins were struck in Canada (the world's largest nickel producer at the time) during non-war years from 1922 to 1981; the metal content made these coins magnetic. During the war years 1942–45, most or all nickel was removed from Canadian and US coins to save it for making armor. Canada used 99.9% nickel from 1968 in its higher-value coins until 2000.Switzerland
Coins of nearly pure nickel were first used in 1881 in Switzerland.United Kingdom
Birmingham forged nickel coins in for trading in Malaysia.United States
 In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the
In the United States, the term "nickel" or "nick" originally applied to the copper-nickel Flying Eagle cent, which replaced copper with 12% nickel 1857–58, then the Indian Head cent of the same alloy from 1859 to 1864. Still later, in 1865, the term designated the three-cent nickel
The copper-nickel three-cent piece, often called a three-cent nickel piece or three-cent nickel, was designed by US Mint Chief Engraver James B. Longacre and struck by the United States Bureau of the Mint from 1865 to 1889. It was initially po ...
, with nickel increased to 25%. In 1866, the five-cent shield nickel (25% nickel, 75% copper) appropriated the designation, which has been used ever since for the subsequent 5-cent pieces. This alloy proportion is not ferromagnetic.
The US nickel coin contains of nickel, which at the April 2007 price was worth 6.5 cents, along with 3.75 grams of copper worth about 3 cents, with a total metal value of more than 9 cents. Since the face value of a nickel is 5 cents, this made it an attractive target for melting by people wanting to sell the metals at a profit. The United States Mint
The United States Mint is a bureau of the Department of the Treasury responsible for producing coinage for the United States to conduct its trade and commerce, as well as controlling the movement of bullion. It does not produce paper money; tha ...
, anticipating this practice, implemented new interim rules on December 14, 2006, subject to public comment for 30 days, which criminalized the melting and export of cents and nickels. Violators can be punished with a fine of up to $10,000 and/or a maximum of five years in prison. As of September 19, 2013, the melt value of a US nickel (copper and nickel included) is $0.045 (90% of the face value).
Current use
In the 21st century, the high price of nickel has led to some replacement of the metal in coins around the world. Coins still made with nickel alloys include one- and two- euro coins, 5¢, 10¢, 25¢, 50¢, and $1 U.S. coins, and 20p, 50p, £1, and £2 UK coins. From 2012 on the nickel-alloy used for 5p and 10p UK coins was replaced with nickel-plated steel. This ignited a public controversy regarding the problems of people withnickel allergy
Nickel allergy or nickel allergic contact dermatitis (Ni-ACD) is a form of allergic contact dermatitis (ACD) caused by exposure to the chemical element nickel. It typically causes a rash that is red and itchy and that may be bumpy or scaly. The ...
.
World production
Philippines
The Philippines (; fil, Pilipinas, links=no), officially the Republic of the Philippines ( fil, Republika ng Pilipinas, links=no),
* bik, Republika kan Filipinas
* ceb, Republika sa Pilipinas
* cbk, República de Filipinas
* hil, Republ ...
(370,000 t), Russia
Russia (, , ), or the Russian Federation, is a transcontinental country spanning Eastern Europe and Northern Asia. It is the largest country in the world, with its internationally recognised territory covering , and encompassing one-eig ...
(250,000 t), New Caledonia (France
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of overseas regions and territories in the Americas and the Atlantic, Pacific and Indian Oceans. Its metropolitan area ...
) (190,000 t), Australia (160,000 t) and Canada
Canada is a country in North America. Its ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, covering over , making it the world's second-largest country by tot ...
(130,000 t) are the largest producers as of 2021. The largest nickel deposits in non-Russian Europe are in Finland and Greece
Greece,, or , romanized: ', officially the Hellenic Republic, is a country in Southeast Europe. It is situated on the southern tip of the Balkans, and is located at the crossroads of Europe, Asia, and Africa. Greece shares land borders ...
. Identified land-based sources averaging at least 1% nickel contain at least 130 million tonnes of nickel. About 60% is in laterites and 40% is in sulfide deposits. Also, extensive nickel sources are found in the depths of the Pacific Ocean, especially in an area called the Clarion Clipperton Zone in the form of polymetallic nodules peppering the seafloor
The seabed (also known as the seafloor, sea floor, ocean floor, and ocean bottom) is the bottom of the ocean
The ocean (also the sea or the world ocean) is the body of salt water that covers approximately 70.8% of the surface of Earth an ...
at 3.5–6 km below sea level
Mean sea level (MSL, often shortened to sea level) is an average surface level of one or more among Earth's coastal bodies of water from which heights such as elevation may be measured. The global MSL is a type of vertical datuma standardise ...
. These nodules are composed of numerous rare-earth metals and are estimated to be 1.7% nickel. With advances in science and engineering
Engineering is the use of scientific principles to design and build machines, structures, and other items, including bridges, tunnels, roads, vehicles, and buildings. The discipline of engineering encompasses a broad range of more speciali ...
, regulation is currently being set in place by the International Seabed Authority
The International Seabed Authority (ISA) (french: Autorité internationale des fonds marins) is a Kingston, Jamaica-based intergovernmental body of 167 member states and the European Union established under the 1982 UN Convention on the Law of ...
to ensure that these nodules are collected in an environmentally conscientious manner while adhering to the United Nations
The United Nations (UN) is an intergovernmental organization whose stated purposes are to maintain international peace and security, develop friendly relations among nations, achieve international cooperation, and be a centre for harmoniz ...
Sustainable Development Goals.
The one place in the United States where nickel has been profitably mined is Riddle, Oregon
Riddle is a city in Douglas County, Oregon, United States. The population was 1,185 at the 2010 census.
History
Riddle was founded by John Bouseman Riddle, the son of William H. Riddle, for whom the town was named. William H. Riddle was a native ...
, with several square miles of nickel-bearing garnierite
Garnierite is a general name for a green nickel ore which is found in pockets and veins within weathered and serpentinized ultramafic rocks. It forms by lateritic weathering of ultramafic rocks and occurs in many nickel laterite deposits in the ...
surface deposits. The mine closed in 1987. The Eagle mine project is a new nickel mine in Michigan's Upper Peninsula
The Upper Peninsula of Michigan – also known as Upper Michigan or colloquially the U.P. – is the northern and more elevated of the two major landmasses that make up the U.S. state of Michigan; it is separated from the Lower Peninsula by ...
. Construction was completed in 2013, and operations began in the third quarter of 2014. In the first full year of operation, the Eagle Mine produced 18,000 t.
Production
pyrometallurgical
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable ...
techniques to produce a matte for further refining. Recent advances in hydrometallurgical techniques result in significantly purer metallic nickel product. Most sulfide deposits have traditionally been processed by concentration through a froth flotation
Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic. This is used in mineral processing, paper recycling and waste-water treatment industries. Historically this was first used in the mining industry, wher ...
process followed by pyrometallurgical extraction. In hydrometallurgical processes, nickel sulfide ores are concentrated with flotation (differential flotation if Ni/Fe ratio is too low) and then smelted. The nickel matte is further processed with the Sherritt-Gordon process. First, copper is removed by adding hydrogen sulfide, leaving a concentrate of cobalt and nickel. Then, solvent extraction is used to separate the cobalt and nickel, with the final nickel content greater than 99%.

Electrorefining
A second common refining process is leaching the metal matte into a nickel salt solution, followed by electrowinning the nickel from solution by plating it onto a cathode as electrolytic nickel.Mond process
 The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form
The purest metal is obtained from nickel oxide by the Mond process, which gives a purity of over 99.99%. The process was patented by Ludwig Mond and has been in industrial use since before the beginning of the 20th century. In this process, nickel is reacted with carbon monoxide in the presence of a sulfur catalyst at around 40–80 °C to form nickel carbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-pu ...
. In a similar reaction with iron, iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to ...
can form, though this reaction is slow. If necessary, the nickel may be separated by distillation. Dicobalt octacarbonyl is also formed in nickel distillation as a by-product, but it decomposes to tetracobalt dodecacarbonyl at the reaction temperature to give a non-volatile solid.
Nickel is obtained from nickel carbonyl by one of two processes. It may be passed through a large chamber at high temperatures in which tens of thousands of nickel spheres (pellets) are constantly stirred. The carbonyl decomposes and deposits pure nickel onto the spheres. In the alternate process, nickel carbonyl is decomposed in a smaller chamber at 230 °C to create a fine nickel powder. The byproduct carbon monoxide is recirculated and reused. The highly pure nickel product is known as "carbonyl nickel".
Market value
The market price of nickel surged throughout 2006 and the early months of 2007; , the metal was trading at US$52,300/ tonne or $1.47/oz. The price later fell dramatically; , the metal was trading at $11,000/tonne, or $0.31/oz. During the2022 Russian invasion of Ukraine
On 24 February 2022, in a major escalation of the Russo-Ukrainian War, which began in 2014. The invasion has resulted in tens of thousands of deaths on both sides. It has caused Europe's largest refugee crisis since World War II. ...
, worries about sanctions on Russian nickel exports triggered a short squeeze, causing the price of nickel to quadruple in just two days, reaching US$100,000 per tonne. The London Metal Exchange
The London Metal Exchange (LME) is a futures and forwards exchange with the world's largest market in standarised forward contracts, futures contracts and options on base metals. The exchange also offers contracts on ferrous metals and precious ...
cancelled contracts worth $3.9 billion and suspended nickel trading for over a week. Analyst Andy Home argued that such price shocks are exacerbated by the purity requirements imposed by metal markets: only Grade I (99.8% pure) metal can be used as a commodity on the exchanges, but most of the world's supply is either in ferro-nickel alloys or lower-grade purities.
Applications
 Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9% electroplating, 7% alloy steel, 3% foundries, and 4% other (including batteries).
Nickel is used in many recognizable industrial and consumer products, including stainless steel,
Global use of nickel is currently 68% in stainless steel, 10% in nonferrous alloys, 9% electroplating, 7% alloy steel, 3% foundries, and 4% other (including batteries).
Nickel is used in many recognizable industrial and consumer products, including stainless steel, alnico
Alnico is a family of iron alloys which in addition to iron are composed primarily of aluminium (Al), nickel (Ni), and cobalt (Co), hence the acronym ''al-ni-co''. They also include copper, and sometimes titanium. Alnico alloys are ferromagnetic, ...
magnets, coinage, rechargeable batteries
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or prima ...
(e.g. nickel-iron), electric guitar strings, microphone capsules, plating on plumbing fixtures, and special alloys such as permalloy, elinvar
Elinvar is a nickel–iron–chromium alloy notable for having a modulus of elasticity which does not change much with temperature changes. The name is a contraction of the French ('invariable elasticity'). It was invented by Charles Édouard Guil ...
, and invar
Invar, also known generically as FeNi36 (64FeNi in the US), is a nickel–iron alloy notable for its uniquely low coefficient of thermal expansion (CTE or α). The name ''Invar'' comes from the word ''invariable'', referring to its relative lac ...
. It is used for plating and as a green tint in glass. Nickel is preeminently an alloy metal, and its chief use is in nickel steels and nickel cast irons, in which it typically increases the tensile strength, toughness, and elastic limit. It is widely used in many other alloys, including nickel brasses and bronzes and alloys with copper, chromium, aluminium, lead, cobalt, silver, and gold (Inconel
Inconel is a registered trademark of Special Metals Corporation for a family of austenitic nickel-chromium-based superalloys.
Inconel alloys are oxidation-corrosion-resistant materials well suited for service in extreme environments subjected ...
, Incoloy
Incoloy refers to a range of superalloys now produced by the Special Metals Corporation (SMC) group of companies and created with a trademark by the Inco company in 1952. Originally Inco protected these alloys by patent. In 2000, the SMC published ...
, Monel
Monel is a group of alloys of nickel (from 52 to 67%) and copper, with small amounts of iron, manganese, carbon, and silicon. Monel is not a cupronickel alloy because it has less than 60% copper.
Stronger than pure nickel, Monel alloys are res ...
, Nimonic Nimonic is a registered trademark of Special Metals Corporation that refers to a family of nickel-based high-temperature low creep superalloys. Nimonic alloys typically consist of more than 50% nickel and 20% chromium with additives such as titani ...
).
 Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper stainless steel (i.e., iron) alloys, except in the United States and Canada.
Nickel is an excellent alloying agent for certain precious metals and is used in the
Because nickel is resistant to corrosion, it was occasionally used as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above), but in the later years of the 20th century, it was replaced by cheaper stainless steel (i.e., iron) alloys, except in the United States and Canada.
Nickel is an excellent alloying agent for certain precious metals and is used in the fire assay
A metallurgical assay is a compositional analysis of an ore, metal, or alloy, usually performed in order to test for purity or quality.
Some assay methods are suitable for raw materials; others are more appropriate for finished goods. Raw pre ...
as a collector of platinum group elements
The platinum-group metals (abbreviated as the PGMs; alternatively, the platinoids, platinides, platidises, platinum group, platinum metals, platinum family or platinum-group elements (PGEs)) are six noble, precious metallic elements clustered t ...
(PGE). As such, nickel can fully collect all six PGEs from ores, and can partially collect gold. High-throughput nickel mines may also do PGE recovery (mainly platinum and palladium); examples are Norilsk, Russia and the Sudbury Basin, Canada.
Nickel foam or nickel mesh is used in gas diffusion electrodes for alkaline fuel cells.
Nickel and its alloys are often used as catalysts for hydrogenation reactions. Raney nickel, a finely divided nickel-aluminium alloy, is one common form, though related catalysts are also used, including Raney-type catalysts.
Nickel is naturally magnetostrictive: in the presence of a magnetic field, the material undergoes a small change in length. The magnetostriction
Magnetostriction (cf. electrostriction) is a property of magnetic materials that causes them to change their shape or dimensions during the process of magnetization. The variation of materials' magnetization due to the applied magnetic field chan ...
of nickel is on the order of 50 ppm and is negative, indicating that it contracts.
Nickel is used as a binder in the cemented tungsten carbide or hardmetal industry and used in proportions of 6% to 12% by weight. Nickel makes the tungsten carbide magnetic and adds corrosion-resistance to the cemented parts, though the hardness is less than those with cobalt binder.
, with half-life 100.1 years, is useful in krytron
The krytron is a cold-cathode gas-filled tube intended for use as a very high-speed switch, somewhat similar to the thyratron. It consists of a sealed glass tube with four electrodes. A small triggering pulse on the grid electrode switches the ...
devices as a beta particle (high-speed electron) emitter to make ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecul ...
by the keep-alive electrode more reliable. It is being investigated as a power source for betavoltaic batteries.
Around 27% of all nickel production is used for engineering, 10% for building and construction, 14% for tubular products, 20% for metal goods, 14% for transport, 11% for electronic goods, and 5% for other uses.
Raney nickel is widely used for hydrogenation of unsaturated oils to make margarine, and substandard margarine and leftover oil may contain nickel as a contaminant
Contamination is the presence of a constituent, impurity, or some other undesirable element that spoils, corrupts, infects, makes unfit, or makes inferior a material, physical body, natural environment, workplace, etc.
Types of contamination
Wi ...
. Forte et al. found that type 2 diabetic patients have 0.89 ng/mL of Ni in the blood relative to 0.77 ng/mL in control subjects.
Biological role
It was not recognized until the 1970s, but nickel is known to play an important role in the biology of some plants,bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
, archaea, and fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
. Nickel enzymes such as urease are considered virulence factors in some organisms. Urease catalyzes hydrolysis of urea to form ammonia and carbamate. NiFe hydrogenases can catalyze oxidation of to form protons and electrons; and also the reverse reaction, the reduction of protons to form hydrogen gas. A nickel-tetrapyrrole coenzyme, cofactor F430
F430 is the cofactor (sometimes called the coenzyme) of the enzyme methyl coenzyme M reductase (MCR).
MCR catalyzes the reaction that releases methane in the final step of methanogenesis:
: + HS–CoB → + CoB–S–S–CoM
It is found ...
, is present in methyl coenzyme M reductase, which can catalyze the formation of methane, or the reverse reaction, in methanogenic archaea (in +1 oxidation state). One of the carbon monoxide dehydrogenase enzymes consists of an Fe-Ni- S cluster. Other nickel-bearing enzymes include a rare bacterial class of superoxide dismutase and glyoxalase I
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal.
:('' ...
enzymes in bacteria and several eukaryotic trypanosomal parasites (in other organisms, including yeast and mammals, this enzyme contains divalent ).
Dietary nickel may affect human health through infections by nickel-dependent bacteria, but nickel may also be an essential nutrient for bacteria living in the large intestine, in effect functioning as a prebiotic. The US Institute of Medicine has not confirmed that nickel is an essential nutrient for humans, so neither a Recommended Dietary Allowance (RDA) nor an Adequate Intake have been established. The tolerable upper intake level
The Dietary Reference Intake (DRI) is a system of nutrition recommendations from the National Academy of Medicine (NAM) of the National Academies (United States). It was introduced in 1997 in order to broaden the existing guidelines known as Re ...
of dietary nickel is 1 mg/day as soluble nickel salts. Estimated dietary intake is 70 to 100 µg/day; less than 10% is absorbed. What is absorbed is excreted in urine. Relatively large amounts of nickel – comparable to the estimated average ingestion above – leach into food cooked in stainless steel. For example, the amount of nickel leached after 10 cooking cycles into one serving of tomato sauce averages 88 µg.
Nickel released from Siberian Traps
The Siberian Traps (russian: Сибирские траппы, Sibirskiye trappy) is a large region of volcanic rock, known as a large igneous province, in Siberia, Russia. The massive eruptive event that formed the traps is one of the largest ...
volcanic eruptions is suspected of helping the growth of ''Methanosarcina
''Methanosarcina'' is a genus of euryarchaeote archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as ...
'', a genus of euryarchaeote archaea that produced methane in the Permian–Triassic extinction event, the biggest known mass extinction.
Toxicity
The major source of nickel exposure is oral consumption, as nickel is essential to plants. Typical background concentrations of nickel do not exceed 20 ng/m in air, 100 mg/kg in soil, 10 mg/kg in vegetation, 10 μg/L in freshwater and 1 μg/L in seawater. Environmental concentrations may be increased by human pollution. For example, nickel-platedfaucet
A tap (also spigot or faucet: see #Nomenclature, usage variations) is a valve controlling the release of a liquid or gas.
Nomenclature
United Kingdom
* Tap is used in the United Kingdom and most of the Commonwealth of Nations, Commonwealth ...
s may contaminate water and soil; mining and smelting may dump nickel into wastewater
Wastewater is water generated after the use of freshwater, raw water, drinking water or saline water in a variety of deliberate applications or processes. Another definition of wastewater is "Used water from any combination of domestic, industrial ...
; nickel–steel alloy cookware and nickel-pigmented dishes may release nickel into food. Air may be polluted by nickel ore refining and fossil fuel combustion. Humans may absorb nickel directly from tobacco smoke
Tobacco smoke is a sooty aerosol produced by the incomplete combustion of tobacco during the smoking of cigarettes and other tobacco products. Temperatures in burning cigarettes range from about 400 °C between puffs to about 900 °C d ...
and skin contact with jewelry, shampoos, detergents, and coins. A less common form of chronic exposure is through hemodialysis
Hemodialysis, also spelled haemodialysis, or simply dialysis, is a process of purifying the blood of a person whose kidneys are not working normally. This type of dialysis achieves the extracorporeal removal of waste products such as creatinin ...
as traces of nickel ions may be absorbed into the plasma from the chelating
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
action of albumin.
The average daily exposure is not a threat to human health. Most nickel absorbed by humans is removed by the kidneys and passed out of the body through urine or is eliminated through the gastrointestinal tract without being absorbed. Nickel is not a cumulative poison, but larger doses or chronic inhalation exposure may be toxic, even carcinogenic, and constitute an occupational hazard
An occupational hazard is a hazard experienced in the workplace. This encompasses many types of hazards, including chemical hazards, biological hazards (biohazards), psychosocial hazards, and physical hazards. In the United States, the Nation ...
.
Nickel compounds are classified as human carcinogensIARC (2012)"Nickel and nickel compounds"
in ''IARC Monogr Eval Carcinog Risks Hum''. Volume 100C. pp. 169–218..Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006 J L 353, 31.12.2008, p. 1br>Annex VI
. Accessed July 13, 2017.Globally Harmonised System of Classification and Labelling of Chemicals (GHS)
, 5th ed., United Nations, New York and Geneva, 2013..National Toxicology Program. (2016)
, 14th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.. based on increased respiratory cancer risks observed in epidemiological studies of sulfidic ore refinery workers. This is supported by the positive results of the NTP bioassays with Ni sub-sulfide and Ni oxide in rats and mice. The human and animal data consistently indicate a lack of carcinogenicity via the oral route of exposure and limit the carcinogenicity of nickel compounds to respiratory tumours after inhalation. Nickel metal is classified as a suspect carcinogen; there is consistency between the absence of increased respiratory cancer risks in workers predominantly exposed to metallic nickel and the lack of respiratory tumours in a rat lifetime inhalation carcinogenicity study with nickel metal powder. In the rodent inhalation studies with various nickel compounds and nickel metal, increased lung inflammations with and without bronchial lymph node hyperplasia or fibrosis were observed. In rat studies, oral ingestion of water-soluble nickel salts can trigger perinatal mortality in pregnant animals. Whether these effects are relevant to humans is unclear as epidemiological studies of highly exposed female workers have not shown adverse developmental toxicity effects. People can be exposed to nickel in the workplace by inhalation, ingestion, and contact with skin or eye. The Occupational Safety and Health Administration (OSHA) has set the legal limit ( permissible exposure limit) for the workplace at 1 mg/m per 8-hour workday, excluding nickel carbonyl. The
National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the C ...
(NIOSH) sets the recommended exposure limit
A recommended exposure limit (REL) is an occupational exposure limit that has been recommended by the United States National Institute for Occupational Safety and Health. The REL is a level that NIOSH believes would be protective of worker safet ...
(REL) at 0.015 mg/m per 8-hour workday. At 10 mg/m, nickel is immediately dangerous to life and health. Nickel carbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-pu ...
is an extremely toxic gas. The toxicity of metal carbonyls is a function of both the toxicity of the metal and the off-gassing of carbon monoxide from the carbonyl functional groups; nickel carbonyl is also explosive in air.
Sensitized persons may show a skin contact allergy to nickel known as a contact dermatitis. Highly sensitized persons may also react to foods with high nickel content. Patients with pompholyx may also be sensitive to nickel. Nickel is the top confirmed contact allergen worldwide, partly due to its use in jewelry for pierced ears. Nickel allergies affecting pierced ears are often marked by itchy, red skin. Many earrings are now made without nickel or with low-release nickel to address this problem. The amount allowed in products that contact human skin is now regulated by the European Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been de ...
. In 2002, researchers found that the nickel released by 1 and 2 euro coins, far exceeded those standards. This is believed to be due to a galvanic reaction. Nickel was voted Allergen of the Year in 2008 by the American Contact Dermatitis Society. In August 2015, the American Academy of Dermatology adopted a position statement on the safety of nickel: "Estimates suggest that contact dermatitis, which includes nickel sensitization, accounts for approximately $1.918 billion and affects nearly 72.29 million people."Position Statement on Nickel Sensitivity. American Academy of Dermatology(August 22, 2015) Reports show that both the nickel-induced activation of hypoxia-inducible factor (HIF-1) and the up-regulation of hypoxia-inducible genes are caused by depletion of intracellular ascorbate. The addition of ascorbate to the culture medium increased the intracellular ascorbate level and reversed both the metal-induced stabilization of HIF-1- and HIF-1α-dependent gene expression.
References
External links
Nickel
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
CDC – Nickel – NIOSH Workplace Safety and Health Topic
An occupational hygiene assessment of dermal nickel exposures in primary production industries
by GW Hughson.
Institute of Occupational Medicine The Institute of Occupational Medicine (IOM) was founded in 1969 by the National Coal Board (NCB) as an independent charity in the UK and retains this charitable purpose and status today. The "Institute" has a subsidiary, IOM Consulting Limited, whi ...
Research Report TM/04/05
An occupational hygiene assessment of dermal nickel exposures in primary production and primary user industries. Phase 2 Report
by GW Hughson.
Institute of Occupational Medicine The Institute of Occupational Medicine (IOM) was founded in 1969 by the National Coal Board (NCB) as an independent charity in the UK and retains this charitable purpose and status today. The "Institute" has a subsidiary, IOM Consulting Limited, whi ...
Research Report TM/05/06"The metal that brought you cheap flights"
BBC News
BBC News is an operational business division of the British Broadcasting Corporation (BBC) responsible for the gathering and broadcasting of news and current affairs in the UK and around the world. The department is the world's largest broad ...
{{Authority control
Chemical elements
Transition metals
Dietary minerals
Ferromagnetic materials
IARC Group 2B carcinogens
Native element minerals
Chemical elements with face-centered cubic structure