lambda phage on:
[Wikipedia]
[Google]
[Amazon]
''Enterobacteria phage λ'' (lambda phage, coliphage λ, officially ''Escherichia virus Lambda'') is a bacterial virus, or
 The virus particle consists of a head and a tail that can have tail fibers. The whole particle consists of 12–14 different proteins with more than 1000 protein molecules total and one DNA molecule located in the phage head. However, it is still not entirely clear whether the L and M proteins are part of the virion. All characterized lambdoid phages possess an N protein-mediated transcription antitermination mechanism, with the exception of phage HK022
The virus particle consists of a head and a tail that can have tail fibers. The whole particle consists of 12–14 different proteins with more than 1000 protein molecules total and one DNA molecule located in the phage head. However, it is still not entirely clear whether the L and M proteins are part of the virion. All characterized lambdoid phages possess an N protein-mediated transcription antitermination mechanism, with the exception of phage HK022
 The
The
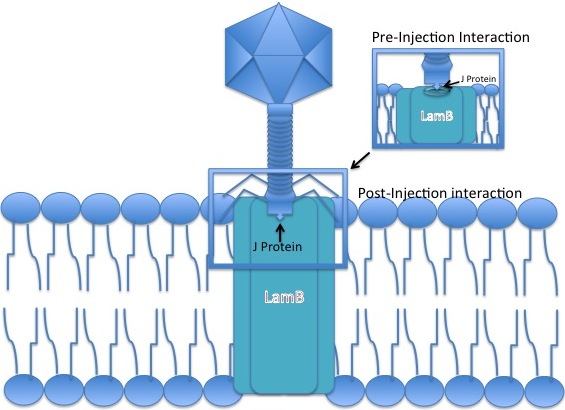 Lambda phage is a non-contractile tailed phage, meaning during an infection event it cannot 'force' its DNA through a bacterial cell membrane. It must instead use an existing pathway to invade the host cell, having evolved the tip of its tail to interact with a specific pore to allow entry of its DNA to the hosts.
# Bacteriophage Lambda binds to an ''E. coli'' cell by means of its J protein in the tail tip. The J protein interacts with the maltose outer membrane porin (the product of the ''lamB'' gene) of ''E. coli'', a porin molecule, which is part of the maltose operon.
# The linear phage genome is injected through the outer membrane.
# The DNA passes through the mannose permease complex in the inner membrane (encoded by the manXYZ genes) and immediately circularises using the ''cos'' sites, 12-base G-C-rich cohesive "sticky ends". The single-strand viral DNA ends are ligated by host
Lambda phage is a non-contractile tailed phage, meaning during an infection event it cannot 'force' its DNA through a bacterial cell membrane. It must instead use an existing pathway to invade the host cell, having evolved the tip of its tail to interact with a specific pore to allow entry of its DNA to the hosts.
# Bacteriophage Lambda binds to an ''E. coli'' cell by means of its J protein in the tail tip. The J protein interacts with the maltose outer membrane porin (the product of the ''lamB'' gene) of ''E. coli'', a porin molecule, which is part of the maltose operon.
# The linear phage genome is injected through the outer membrane.
# The DNA passes through the mannose permease complex in the inner membrane (encoded by the manXYZ genes) and immediately circularises using the ''cos'' sites, 12-base G-C-rich cohesive "sticky ends". The single-strand viral DNA ends are ligated by host  # Host
# Host  # Cro binds to ''OR3'', preventing access to the ''PRM'' promoter, preventing expression of the ''cI'' gene. N binds to the two ''Nut'' (N utilisation) sites, one in the ''N'' gene in the ''PL'' reading frame, and one in the ''cro'' gene in the ''PR'' reading frame.
# The N protein is an
# Cro binds to ''OR3'', preventing access to the ''PRM'' promoter, preventing expression of the ''cI'' gene. N binds to the two ''Nut'' (N utilisation) sites, one in the ''N'' gene in the ''PL'' reading frame, and one in the ''cro'' gene in the ''PR'' reading frame.
# The N protein is an
 This is the lifecycle that the phage follows following most infections, where the cII protein does not reach a high enough concentration due to degradation, so does not activate its promoters.
# The 'late early' transcripts continue being written, including ''xis'', ''int'', ''Q'' and genes for replication of the lambda genome (''OP''). Cro dominates the repressor site (see "Repressor" section), repressing synthesis from the ''PRM'' promoter (which is a promoter of the lysogenic cycle).
# The O and P proteins initiate replication of the phage chromosome (see "Lytic Replication").
# Q, another
This is the lifecycle that the phage follows following most infections, where the cII protein does not reach a high enough concentration due to degradation, so does not activate its promoters.
# The 'late early' transcripts continue being written, including ''xis'', ''int'', ''Q'' and genes for replication of the lambda genome (''OP''). Cro dominates the repressor site (see "Repressor" section), repressing synthesis from the ''PRM'' promoter (which is a promoter of the lysogenic cycle).
# The O and P proteins initiate replication of the phage chromosome (see "Lytic Replication").
# Q, another
 Leftward transcription expresses the ''gam'', ''red'', ''xis'', and ''int'' genes. Gam and red proteins are involved in recombination. Gam is also important in that it inhibits the host RecBCD nuclease from degrading the 3’ ends in rolling circle replication. Int and xis are integration and excision proteins vital to lysogeny.
Leftward transcription expresses the ''gam'', ''red'', ''xis'', and ''int'' genes. Gam and red proteins are involved in recombination. Gam is also important in that it inhibits the host RecBCD nuclease from degrading the 3’ ends in rolling circle replication. Int and xis are integration and excision proteins vital to lysogeny.
 * Lysogeny is maintained solely by cI. cI represses transcription from ''PL'' and ''PR'' while upregulating and controlling its own expression from ''PRM''. It is therefore the only protein expressed by lysogenic phage.
* Lysogeny is maintained solely by cI. cI represses transcription from ''PL'' and ''PR'' while upregulating and controlling its own expression from ''PRM''. It is therefore the only protein expressed by lysogenic phage.
 * This is coordinated by the ''PL'' and ''PR'' operators. Both operators have three binding sites for cI: ''OL1'', ''OL2'', and ''OL3'' for ''PL'', and ''OR1'', ''OR2'' and ''OR3'' for ''PR''.
* cI binds most favorably to ''OR1''; binding here inhibits transcription from ''PR''. As cI easily dimerises, the binding of cI to ''OR1'' greatly increases the affinity of the binding of cI to ''OR2'', and this happens almost immediately after ''OR1'' binding. This activates transcription in the other direction from ''PRM'', as the N terminal domain of cI on ''OR2'' tightens the binding of RNA polymerase to ''PRM'' and hence cI stimulates its own transcription. When it is present at a much higher concentration, it also binds to ''OR3'', inhibiting transcription from ''PRM'', thus regulating its own levels in a negative feedback loop.
* cI binding to the ''PL'' operator is very similar, except that it has no direct effect on cI transcription. As an additional repression of its own expression, however, cI dimers bound to ''OR3'' and ''OL3'' bend the DNA between them to tetramerise.
* The presence of cI causes immunity to superinfection by other lambda phages, as it will inhibit their ''PL'' and ''PR'' promoters.
* This is coordinated by the ''PL'' and ''PR'' operators. Both operators have three binding sites for cI: ''OL1'', ''OL2'', and ''OL3'' for ''PL'', and ''OR1'', ''OR2'' and ''OR3'' for ''PR''.
* cI binds most favorably to ''OR1''; binding here inhibits transcription from ''PR''. As cI easily dimerises, the binding of cI to ''OR1'' greatly increases the affinity of the binding of cI to ''OR2'', and this happens almost immediately after ''OR1'' binding. This activates transcription in the other direction from ''PRM'', as the N terminal domain of cI on ''OR2'' tightens the binding of RNA polymerase to ''PRM'' and hence cI stimulates its own transcription. When it is present at a much higher concentration, it also binds to ''OR3'', inhibiting transcription from ''PRM'', thus regulating its own levels in a negative feedback loop.
* cI binding to the ''PL'' operator is very similar, except that it has no direct effect on cI transcription. As an additional repression of its own expression, however, cI dimers bound to ''OR3'' and ''OL3'' bend the DNA between them to tetramerise.
* The presence of cI causes immunity to superinfection by other lambda phages, as it will inhibit their ''PL'' and ''PR'' promoters.
 The classic induction of a lysogen involved irradiating the infected cells with UV light. Any situation where a lysogen undergoes DNA damage or the
The classic induction of a lysogen involved irradiating the infected cells with UV light. Any situation where a lysogen undergoes DNA damage or the 
 The repressor found in the phage lambda is a notable example of the level of control possible over gene expression by a very simple system. It forms a 'binary switch' with two genes under mutually exclusive expression, as discovered by Barbara J. Meyer.
The repressor found in the phage lambda is a notable example of the level of control possible over gene expression by a very simple system. It forms a 'binary switch' with two genes under mutually exclusive expression, as discovered by Barbara J. Meyer.
 The lambda repressor gene system consists of (from left to right on the chromosome):
* ''cI'' gene
* OR3
* OR2
* OR1
* ''cro'' gene
The lambda repressor is a self assembling dimer also known as the
The lambda repressor gene system consists of (from left to right on the chromosome):
* ''cI'' gene
* OR3
* OR2
* OR1
* ''cro'' gene
The lambda repressor is a self assembling dimer also known as the
File:Viral DNA setup.svg, Some base pairs with serve a dual function with promoter and operator for either cl and cro proteins.
File:Polymerase ON.svg, Protein cl turned ON, with repressor bound to OR2 polymerase binding is increased and turn OFF OR1.
File:Repressor concentration.svg, Lysogen repression all 3 sites bound is a low occurrence due to OR3 weak binding affinity. OR1 repression increases binding affinity to OR2 due to repressor-repressor interaction. Increased concentrations of repressor increase binding.
 An important distinction here is that between the two decisions; lysogeny and lysis on infection, and continuing lysogeny or lysis from a prophage. The latter is determined solely by the activation of RecA in the SOS response of the cell, as detailed in the section on induction. The former will also be affected by this; a cell undergoing an SOS response will always be lysed, as no cI protein will be allowed to build up. However, the initial lytic/lysogenic decision on infection is also dependent on the cII and cIII proteins.
In cells with sufficient nutrients, protease activity is high, which breaks down cII. This leads to the lytic lifestyle. In cells with limited nutrients, protease activity is low, making cII stable. This leads to the lysogenic lifestyle. cIII appears to stabilize cII, both directly and by acting as a competitive inhibitor to the relevant proteases. This means that a cell "in trouble", i.e. lacking in nutrients and in a more dormant state, is more likely to lysogenise. This would be selected for because the phage can now lie dormant in the bacterium until it falls on better times, and so the phage can create more copies of itself with the additional resources available and with the more likely proximity of further infectable cells.
A full biophysical model for lambda's lysis-lysogeny decision remains to be developed. Computer modeling and simulation suggest that random processes during infection drive the selection of lysis or lysogeny within individual cells. However, recent experiments suggest that physical differences among cells, that exist prior to infection, predetermine whether a cell will lyse or become a lysogen.
An important distinction here is that between the two decisions; lysogeny and lysis on infection, and continuing lysogeny or lysis from a prophage. The latter is determined solely by the activation of RecA in the SOS response of the cell, as detailed in the section on induction. The former will also be affected by this; a cell undergoing an SOS response will always be lysed, as no cI protein will be allowed to build up. However, the initial lytic/lysogenic decision on infection is also dependent on the cII and cIII proteins.
In cells with sufficient nutrients, protease activity is high, which breaks down cII. This leads to the lytic lifestyle. In cells with limited nutrients, protease activity is low, making cII stable. This leads to the lysogenic lifestyle. cIII appears to stabilize cII, both directly and by acting as a competitive inhibitor to the relevant proteases. This means that a cell "in trouble", i.e. lacking in nutrients and in a more dormant state, is more likely to lysogenise. This would be selected for because the phage can now lie dormant in the bacterium until it falls on better times, and so the phage can create more copies of itself with the additional resources available and with the more likely proximity of further infectable cells.
A full biophysical model for lambda's lysis-lysogeny decision remains to be developed. Computer modeling and simulation suggest that random processes during infection drive the selection of lysis or lysogeny within individual cells. However, recent experiments suggest that physical differences among cells, that exist prior to infection, predetermine whether a cell will lyse or become a lysogen.
''Microbiology and Molecular Biology Reviews''
Online overview of lambda
(illustrates genes active at all stages in lifecycle)
(illustrates infection and lytic/lysogenic pathways with some protein and transcription detail)
Time-lapse microscopy video
from MIT showing both lysis and lysogeny by phage lambda
Lambda Phage Life cycle
(basic visual demonstration of Lambda bacteriophage life cycle)
Lambda Phage genome in GenBank
Lambda Phage Reference Proteome from UniProt
Lambda Phage Protein Structures in NCBI
(3D display of protein structures for bacteriophage Lambda) {{DEFAULTSORT:Lambda Phage Genetics techniques Model organisms Siphoviridae
bacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteri ...
, that infects the bacterial species ''Escherichia coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
'' (''E. coli''). It was discovered by Esther Lederberg
Esther Miriam Zimmer Lederberg (December 18, 1922 – November 11, 2006) was an American microbiologist and a pioneer of bacterial genetics. She discovered the bacterial virus λ and the bacterial fertility factor F, devised the first impl ...
in 1950. The wild type of this virus has a temperate
In geography, the temperate climates of Earth occur in the middle latitudes (23.5° to 66.5° N/S of Equator), which span between the tropics and the polar regions of Earth. These zones generally have wider temperature ranges throughout t ...
life cycle that allows it to either reside within the genome
In the fields of molecular biology and genetics, a genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding ge ...
of its host through lysogeny
Lysogeny, or the lysogenic cycle, is one of two cycles of viral reproduction (the lytic cycle being the other). Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome or formation of a circu ...
or enter into a lytic
The lytic cycle ( ) is one of the two cycles of viral reproduction (referring to bacterial viruses or bacteriophages), the other being the lysogenic cycle. The lytic cycle results in the destruction of the infected cell and its membrane. Bacter ...
phase, during which it kills and lyses the cell to produce offspring. Lambda strains, mutated at specific sites, are unable to lysogenize cells; instead, they grow and enter the lytic cycle after superinfecting an already lysogenized cell.
The phage particle consists of a head (also known as a capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or ma ...
), a tail, and tail fibers (see image of virus below). The head contains the phage's double-strand linear DNA genome. During infection, the phage particle recognizes and binds to its host, ''E. coli'', causing DNA in the head of the phage to be ejected through the tail into the cytoplasm of the bacterial cell. Usually, a "lytic cycle
The lytic cycle ( ) is one of the two cycles of viral reproduction (referring to bacterial viruses or bacteriophages), the other being the lysogenic cycle. The lytic cycle results in the destruction of the infected cell and its membrane. Bacter ...
" ensues, where the lambda DNA is replicated and new phage particles are produced within the cell. This is followed by cell lysis
Lysis ( ) is the breaking down of the membrane of a cell, often by viral, enzymic, or osmotic (that is, "lytic" ) mechanisms that compromise its integrity. A fluid containing the contents of lysed cells is called a ''lysate''. In molecular bio ...
, releasing the cell contents, including virions that have been assembled, into the environment. However, under certain conditions, the phage DNA may integrate itself into the host cell chromosome in the lysogenic
Lysogeny, or the lysogenic cycle, is one of two cycles of viral reproduction (the lytic cycle being the other). Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome or formation of a circu ...
pathway. In this state, the λ DNA is called a prophage and stays resident within the host's genome
In the fields of molecular biology and genetics, a genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding ge ...
without apparent harm to the host. The host is termed a lysogen
A lysogen or lysogenic bacterium is a bacterial cell which can produce and transfer the ability to produce a phage. A prophage is either integrated into the host bacteria's chromosome or more rarely exists as a stable plasmid within the host cell ...
when a prophage is present. This prophage may enter the lytic cycle when the lysogen enters a stressed condition.
Anatomy
genome
In the fields of molecular biology and genetics, a genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding ge ...
contains 48,502 base pairs of double-stranded, linear DNA, with 12-base single-strand segments at both 5' ends.Campbell, A.M. Bacteriophages. In: Neidhardt, FC et al. (1996) ''Escherichia coli'' and ''Salmonella typhimurium'': Cellular and Molecular Biology (ASM Press, Washington, DC) These two single-stranded segments are the "sticky ends" of what is called the ''cos'' site. The ''cos'' site circularizes the DNA in the host cytoplasm. In its circular form, the phage genome, therefore, is 48,502 base pairs in length. The lambda genome can be inserted into the '' E. coli'' chromosome and is then called a prophage. See section below for details.
Life cycle
Infection
 Lambda phage is a non-contractile tailed phage, meaning during an infection event it cannot 'force' its DNA through a bacterial cell membrane. It must instead use an existing pathway to invade the host cell, having evolved the tip of its tail to interact with a specific pore to allow entry of its DNA to the hosts.
# Bacteriophage Lambda binds to an ''E. coli'' cell by means of its J protein in the tail tip. The J protein interacts with the maltose outer membrane porin (the product of the ''lamB'' gene) of ''E. coli'', a porin molecule, which is part of the maltose operon.
# The linear phage genome is injected through the outer membrane.
# The DNA passes through the mannose permease complex in the inner membrane (encoded by the manXYZ genes) and immediately circularises using the ''cos'' sites, 12-base G-C-rich cohesive "sticky ends". The single-strand viral DNA ends are ligated by host
Lambda phage is a non-contractile tailed phage, meaning during an infection event it cannot 'force' its DNA through a bacterial cell membrane. It must instead use an existing pathway to invade the host cell, having evolved the tip of its tail to interact with a specific pore to allow entry of its DNA to the hosts.
# Bacteriophage Lambda binds to an ''E. coli'' cell by means of its J protein in the tail tip. The J protein interacts with the maltose outer membrane porin (the product of the ''lamB'' gene) of ''E. coli'', a porin molecule, which is part of the maltose operon.
# The linear phage genome is injected through the outer membrane.
# The DNA passes through the mannose permease complex in the inner membrane (encoded by the manXYZ genes) and immediately circularises using the ''cos'' sites, 12-base G-C-rich cohesive "sticky ends". The single-strand viral DNA ends are ligated by host DNA ligase
DNA ligase is a specific type of enzyme, a ligase, () that facilitates the joining of DNA strands together by catalyzing the formation of a phosphodiester bond. It plays a role in repairing single-strand breaks in duplex DNA in living orga ...
. It is not generally appreciated that the 12 bp lambda cohesive ends were the subject of the first direct nucleotide sequencing of a biological DNA.
 # Host
# Host DNA gyrase
DNA gyrase, or simply gyrase, is an enzyme within the class of topoisomerase and is a subclass of Type II topoisomerases that reduces topological strain in an ATP dependent manner while double-stranded DNA is being unwound by elongating RNA-poly ...
puts negative supercoils in the circular chromosome, causing A-T-rich regions to unwind and drive transcription.
# Transcription starts from the constitutive ''PL'', ''PR'' and ''PR''' promoters producing the 'immediate early' transcripts. At first, these express the ''N'' and ''cro'' genes, producing N, Cro and a short inactive protein.
antiterminator Antitermination is the prokaryotic cell's aid to fix premature termination of RNA synthesis during the transcription of RNA. It occurs when the RNA polymerase ignores the termination signal and continues elongating its transcript until a second s ...
, and functions by engaging the transcribing RNA polymerase
In molecular biology, RNA polymerase (abbreviated RNAP or RNApol), or more specifically DNA-directed/dependent RNA polymerase (DdRP), is an enzyme that synthesizes RNA from a DNA template.
Using the enzyme helicase, RNAP locally opens the ...
at specific sites of the nascently transcribed mRNA. When RNA polymerase
In molecular biology, RNA polymerase (abbreviated RNAP or RNApol), or more specifically DNA-directed/dependent RNA polymerase (DdRP), is an enzyme that synthesizes RNA from a DNA template.
Using the enzyme helicase, RNAP locally opens the ...
transcribes these regions, it recruits N and forms a complex with several host Nus proteins. This complex skips through most termination sequences. The extended transcripts (the 'late early' transcripts) include the ''N'' and ''cro'' genes along with ''cII'' and ''cIII'' genes, and ''xis'', ''int'', ''O'', ''P'' and ''Q'' genes discussed later.
# The cIII protein acts to protect the cII protein from proteolysis by FtsH (a membrane-bound essential ''E''. ''coli'' protease) by acting as a competitive inhibitor. This inhibition can induce a bacteriostatic
A bacteriostatic agent or bacteriostat, abbreviated Bstatic, is a biological or chemical agent that stops bacteria from reproducing, while not necessarily killing them otherwise. Depending on their application, bacteriostatic antibiotics, disinfect ...
state, which favours lysogeny. cIII also directly stabilises the cII protein.
On initial infection, the stability of cII determines the lifestyle of the phage; stable cII will lead to the lysogenic pathway, whereas if cII is degraded the phage will go into the lytic pathway. Low temperature, starvation of the cells and high multiplicity of infection
In microbiology, the multiplicity of infection or MOI is the ratio of agents (e.g. phage or more generally virus, bacteria) to infection targets (e.g. cell). For example, when referring to a group of cells inoculated with virus particles, the MOI ...
(MOI) are known to favor lysogeny (see later discussion).
N antitermination
This occurs without the N protein interacting with the DNA; the protein instead binds to the freshly transcribed mRNA. Nut sites contain 3 conserved "boxes", of which only BoxB is essential. # The boxB RNA sequences are located close to the 5' end of the pL and pR transcripts. When transcribed, each sequence forms a hairpin loop structure that the N protein can bind to. # N protein binds to boxB in each transcript, and contacts the transcribing RNA polymerase via RNA looping. The N-RNAP complex is stabilized by subsequent binding of several host Nus (N utilisation substance) proteins (which include transcription termination/antitermination factors and, bizarrely, a ribosome subunit). # The entire complex (including the bound ''Nut'' site on the mRNA) continues transcription, and can skip through termination sequences.Lytic life cycle
 This is the lifecycle that the phage follows following most infections, where the cII protein does not reach a high enough concentration due to degradation, so does not activate its promoters.
# The 'late early' transcripts continue being written, including ''xis'', ''int'', ''Q'' and genes for replication of the lambda genome (''OP''). Cro dominates the repressor site (see "Repressor" section), repressing synthesis from the ''PRM'' promoter (which is a promoter of the lysogenic cycle).
# The O and P proteins initiate replication of the phage chromosome (see "Lytic Replication").
# Q, another
This is the lifecycle that the phage follows following most infections, where the cII protein does not reach a high enough concentration due to degradation, so does not activate its promoters.
# The 'late early' transcripts continue being written, including ''xis'', ''int'', ''Q'' and genes for replication of the lambda genome (''OP''). Cro dominates the repressor site (see "Repressor" section), repressing synthesis from the ''PRM'' promoter (which is a promoter of the lysogenic cycle).
# The O and P proteins initiate replication of the phage chromosome (see "Lytic Replication").
# Q, another antiterminator Antitermination is the prokaryotic cell's aid to fix premature termination of RNA synthesis during the transcription of RNA. It occurs when the RNA polymerase ignores the termination signal and continues elongating its transcript until a second s ...
, binds to ''Qut'' sites.
# Transcription from the ''PR''' promoter can now extend to produce mRNA for the lysis and the head and tail proteins.
# Structural proteins and phage genomes self-assemble into new phage particles.
# Products of the genes ''S'',''R'', ''Rz'' and ''Rz1'' cause cell lysis. S is a holin
Holins are a diverse group of small proteins produced by dsDNA bacteriophages in order to trigger and control the degradation of the host's cell wall at the end of the lytic cycle. Holins form pores in the host's cell membrane, allowing lysins t ...
, a small membrane protein that, at a time determined by the sequence of the protein, suddenly makes holes in the membrane. R is an endolysin
Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target ...
, an enzyme that escapes through the S holes and cleaves the cell wall. Rz and Rz1 are membrane proteins that form a complex that somehow destroys the outer membrane, after the endolysin has degraded the cell wall. For wild-type lambda, lysis occurs at about 50 minutes after the start of infection and releases around 100 virions.
Rightward transcription
Rightward transcription expresses the ''O'', ''P'' and ''Q'' genes. O and P are responsible for initiating replication, and Q is another antiterminator that allows the expression of head, tail, and lysis genes from ''PR’''.Lytic replication
# For the first few replication cycles, the lambda genome undergoes θ replication (circle-to-circle). # This is initiated at the ''ori'' site located in the ''O'' gene. O protein binds the ''ori'' site, and P protein binds the DnaB subunit of the host replication machinery as well as binding O. This effectively commandeers the host DNA polymerase. # Soon, the phage switches to arolling circle replication
Rolling circle replication (RCR) is a process of unidirectional nucleic acid replication that can rapidly synthesize multiple copies of circular molecules of DNA or RNA, such as plasmids, the genomes of bacteriophages, and the circular RNA ...
similar to that used by phage M13. The DNA is nicked and the 3’ end serves as a primer. Note that this does not release single copies of the phage genome but rather one long molecule with many copies of the genome: a concatemer
A concatemer is a long continuous DNA molecule that contains multiple copies of the same DNA sequence linked in series. These polymeric molecules are usually copies of an entire genome linked end to end and separated by ''cos'' sites (a protein bi ...
.
# These concatemers are cleaved at their ''cos'' sites as they are packaged. Packaging cannot occur from circular phage DNA, only from concatomeric DNA.
Q antitermination
Q is similar to N in its effect: Q binds toRNA polymerase
In molecular biology, RNA polymerase (abbreviated RNAP or RNApol), or more specifically DNA-directed/dependent RNA polymerase (DdRP), is an enzyme that synthesizes RNA from a DNA template.
Using the enzyme helicase, RNAP locally opens the ...
in ''Qut'' sites and the resulting complex can ignore terminators, however the mechanism is very different; the Q protein first associates with a DNA sequence rather than an mRNA sequence.
# The ''Qut'' site is very close to the ''PR’'' promoter, close enough that the σ factor has not been released from the RNA polymerase holoenzyme. Part of the ''Qut'' site resembles the -10 Pribnow box
The Pribnow box (also known as the Pribnow-Schaller box) is a sequence of ''TATAAT'' of six nucleotides (thymine, adenine, thymine, etc.) that is an essential part of a promoter site on DNA for transcription to occur in bacteria.
It is an idea ...
, causing the holoenzyme to pause.
# Q protein then binds and displaces part of the σ factor and transcription re-initiates.
# The head and tail genes are transcribed and the corresponding proteins self-assemble.
Leftward transcription
 Leftward transcription expresses the ''gam'', ''red'', ''xis'', and ''int'' genes. Gam and red proteins are involved in recombination. Gam is also important in that it inhibits the host RecBCD nuclease from degrading the 3’ ends in rolling circle replication. Int and xis are integration and excision proteins vital to lysogeny.
Leftward transcription expresses the ''gam'', ''red'', ''xis'', and ''int'' genes. Gam and red proteins are involved in recombination. Gam is also important in that it inhibits the host RecBCD nuclease from degrading the 3’ ends in rolling circle replication. Int and xis are integration and excision proteins vital to lysogeny.
xis and int regulation of insertion and excision
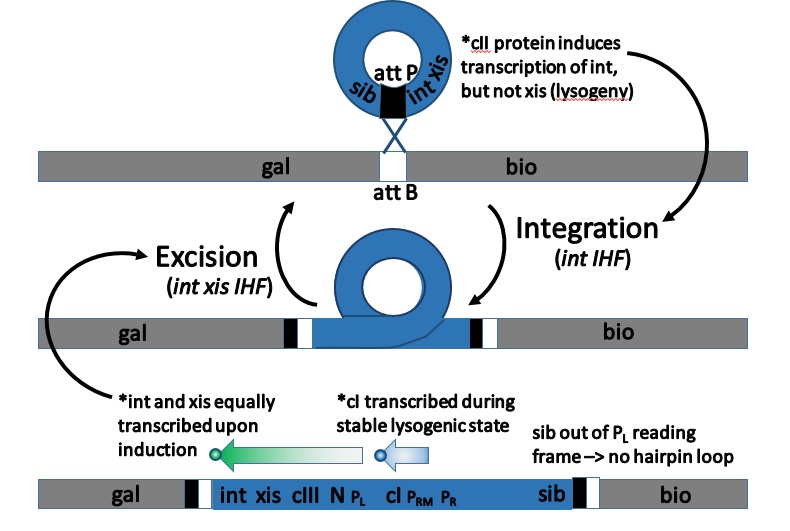
# ''xis'' and ''int'' are found on the same piece of mRNA, so approximately equal concentrations of ''xis'' and ''int'' proteins are produced. This results (initially) in the excision of any inserted genomes from the host genome. # The mRNA from the ''PL'' promoter forms a stable secondary structure with a stem-loop in the ''sib'' section of the mRNA. This targets the 3' (''sib'') end of the mRNA for RNAaseIII degradation, which results in a lower effective concentration of ''int'' mRNA than ''xis'' mRNA (as the ''int'' cistron is nearer to the ''sib'' sequence than the ''xis'' cistron is to the ''sib'' sequence), so a higher concentrations of ''xis'' than ''int'' is observed. # Higher concentrations of ''xis'' than ''int'' result in no insertion or excision of phage genomes, the evolutionarily favoured action - leaving any pre-inserted phage genomes inserted (so reducing competition) and preventing the insertion of the phage genome into the genome of a doomed host.Lysogenic (or lysenogenic) life cycle
The lysogenic lifecycle begins once the cI protein reaches a high enough concentration to activate its promoters, after a small number of infections. # The 'late early' transcripts continue being written, including ''xis'', ''int'', ''Q'' and genes for replication of the lambda genome. # The stabilized cII acts to promote transcription from the ''PRE'', ''PI'' and ''Pantiq'' promoters. # The ''Pantiq'' promoter produces antisense mRNA to the ''Q'' gene message of the ''PR'' promoter transcript, thereby switching off Q production. The ''PRE'' promoter produces antisense mRNA to the cro section of the ''PR'' promoter transcript, turning down cro production, and has a transcript of the ''cI'' gene. This is expressed, turning on cI repressor production. The ''PI'' promoter expresses the ''int'' gene, resulting in high concentrations of Int protein. This int protein integrates the phage DNA into the host chromosome (see "Prophage Integration"). # No Q results in no extension of the ''PR''' promoter's reading frame, so no lytic or structural proteins are made. Elevated levels of int (much higher than that of xis) result in the insertion of the lambda genome into the hosts genome (see diagram). Production of cI leads to the binding of cI to the ''OR1'' and ''OR2'' sites in the ''PR'' promoter, turning off ''cro'' and other early gene expression. cI also binds to the ''PL'' promoter, turning off transcription there too. # Lack of cro leaves the ''OR3'' site unbound, so transcription from the ''PRM'' promoter may occur, maintaining levels of cI. # Lack of transcription from the ''PL'' and ''PR'' promoters leads to no further production of cII and cIII. # As cII and cIII concentrations decrease, transcription from the ''Pantiq'', ''PRE'' and ''PI'' stop being promoted since they are no longer needed. # Only the ''PRM'' and ''PR''' promoters are left active, the former producing cI protein and the latter a short inactive transcript. The genome remains inserted into the host genome in a dormant state. The prophage is duplicated with every subsequent cell division of the host. The phage genes expressed in this dormant state code for proteins that repress expression of other phage genes (such as the structural and lysis genes) in order to prevent entry into the lytic cycle. These repressive proteins are broken down when the host cell is under stress, resulting in the expression of the repressed phage genes. Stress can be fromstarvation
Starvation is a severe deficiency in caloric energy intake, below the level needed to maintain an organism's life. It is the most extreme form of malnutrition. In humans, prolonged starvation can cause permanent organ damage and eventually, dea ...
, poison
Poison is a chemical substance that has a detrimental effect to life. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figuratively, with a broa ...
s (like antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention o ...
), or other factors that can damage or destroy the host. In response to stress, the activated prophage is excised from the DNA of the host cell by one of the newly expressed gene products and enters its lytic pathway.
Prophage integration
The integration of phage λ takes place at a special attachment site in the bacterial and phage genomes, called ''attλ''. The sequence of the bacterial att site is called ''attB'', between the ''gal'' and ''bio'' operons, and consists of the parts B-O-B', whereas the complementary sequence in the circular phage genome is called ''attP'' and consists of the parts P-O-P'. The integration itself is a sequential exchange (see genetic recombination) via a Holliday junction and requires both the phage protein Int and the bacterial protein IHF (''integration host factor''). Both Int and IHF bind to ''attP'' and form an intasome, a DNA-protein-complex designed forsite-specific recombination Site-specific recombination, also known as conservative site-specific recombination, is a type of genetic recombination in which DNA strand exchange takes place between segments possessing at least a certain degree of sequence homology. Enzymes kno ...
of the phage and host DNA. The original B-O-B' sequence is changed by the integration to B-O-P'-phage DNA-P-O-B'. The phage DNA is now part of the host's genome.
Maintenance of lysogeny
 * Lysogeny is maintained solely by cI. cI represses transcription from ''PL'' and ''PR'' while upregulating and controlling its own expression from ''PRM''. It is therefore the only protein expressed by lysogenic phage.
* Lysogeny is maintained solely by cI. cI represses transcription from ''PL'' and ''PR'' while upregulating and controlling its own expression from ''PRM''. It is therefore the only protein expressed by lysogenic phage.
Induction
 The classic induction of a lysogen involved irradiating the infected cells with UV light. Any situation where a lysogen undergoes DNA damage or the
The classic induction of a lysogen involved irradiating the infected cells with UV light. Any situation where a lysogen undergoes DNA damage or the SOS response
The SOS response is a global response to DNA damage in which the cell cycle is arrested and DNA repair and mutagenesis is induced. The system involves the RecA protein (Rad51 in eukaryotes). The RecA protein, stimulated by single-stranded DNA ...
of the host is otherwise stimulated leads to induction.
# The host cell, containing a dormant phage genome, experiences DNA damage due to a high stress environment, and starts to undergo the SOS response
The SOS response is a global response to DNA damage in which the cell cycle is arrested and DNA repair and mutagenesis is induced. The system involves the RecA protein (Rad51 in eukaryotes). The RecA protein, stimulated by single-stranded DNA ...
.
# RecA (a cellular protein) detects DNA damage and becomes activated. It is now RecA*, a highly specific co-protease.
# Normally RecA* binds LexA (a transcription
Transcription refers to the process of converting sounds (voice, music etc.) into letters or musical notes, or producing a copy of something in another medium, including:
Genetics
* Transcription (biology), the copying of DNA into RNA, the fir ...
repressor), activating LexA auto-protease activity, which destroys LexA repressor, allowing production of DNA repair
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA da ...
proteins. In lysogenic cells, this response is hijacked, and RecA* stimulates cI autocleavage. This is because cI mimics the structure of LexA at the autocleavage site.
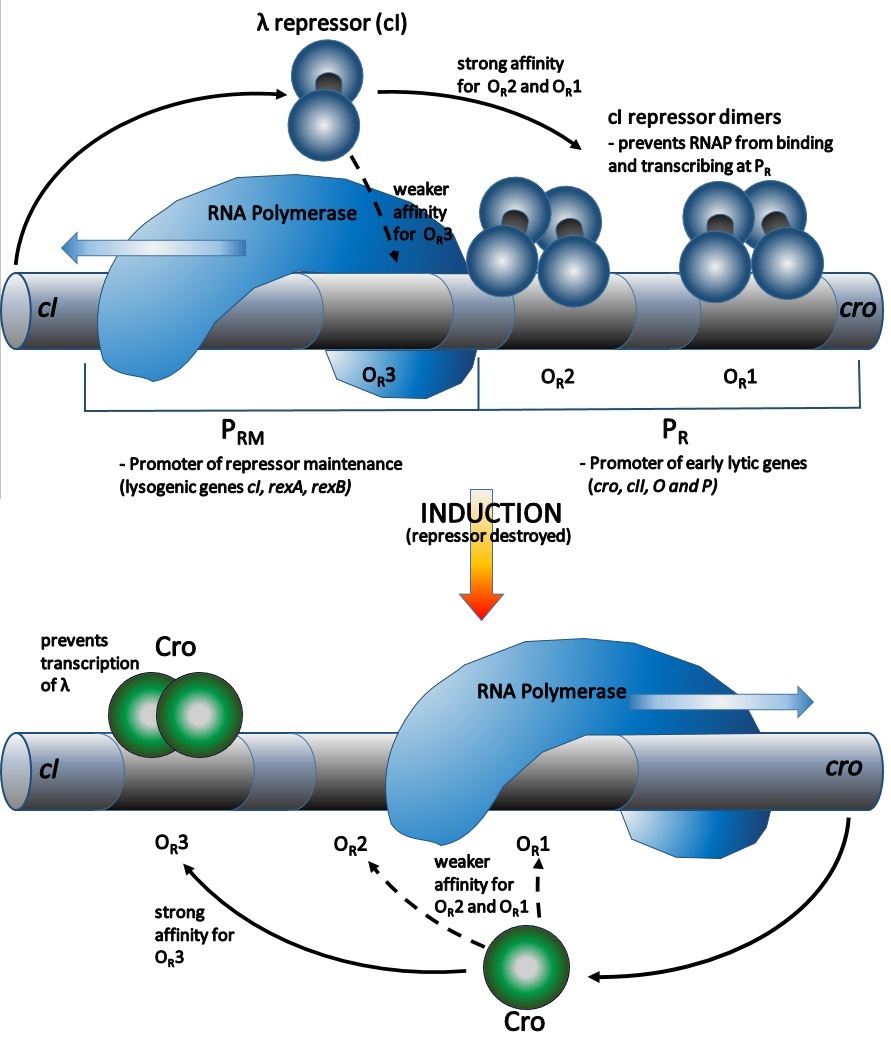
# Cleaved cI can no longer dimerise, and loses its affinity for DNA binding.
# The ''PR'' and ''PL'' promoters are no longer repressed and switch on, and the cell returns to the lytic sequence of expression events (note that cII is not stable in cells undergoing the SOS response). There is however one notable difference.
Control of phage genome excision in induction
# The phage genome is still inserted in the host genome and needs excision for DNA replication to occur. The ''sib'' section beyond the normal ''PL'' promoter transcript is, however, no longer included in this reading frame (see diagram). # No ''sib'' domain on the ''PL'' promoter mRNA results in no hairpin loop on the 3' end, and the transcript is no longer targeted for RNAaseIII degradation. # The new intact transcript has one copy of both ''xis'' and ''int'', so approximately equal concentrations of xis and int proteins are produced. # Equal concentrations of xis and int result in the excision of the inserted genome from the host genome for replication and later phage production.Multiplicity reactivation and prophage reactivation
Multiplicity reactivation (MR) is the process by which multiple viral genomes, each containing inactivating genome damage, interact within an infected cell to form a viable viral genome. MR was originally discovered with phage T4, but was subsequently found in phage λ (as well as in numerous other bacterial and mammalian viruses). MR of phage λ inactivated by UV light depends on the recombination function of either the host or of the infecting phage. Absence of both recombination systems leads to a loss of MR. Survival of UV-irradiated phage λ is increased when the E. coli host is lysogenic for an homologous prophage, a phenomenon termed prophage reactivation. Prophage reactivation in phage λ appears to occur by a recombinational repair process similar to that of MR.Repressor
 The repressor found in the phage lambda is a notable example of the level of control possible over gene expression by a very simple system. It forms a 'binary switch' with two genes under mutually exclusive expression, as discovered by Barbara J. Meyer.
The repressor found in the phage lambda is a notable example of the level of control possible over gene expression by a very simple system. It forms a 'binary switch' with two genes under mutually exclusive expression, as discovered by Barbara J. Meyer.
 The lambda repressor gene system consists of (from left to right on the chromosome):
* ''cI'' gene
* OR3
* OR2
* OR1
* ''cro'' gene
The lambda repressor is a self assembling dimer also known as the
The lambda repressor gene system consists of (from left to right on the chromosome):
* ''cI'' gene
* OR3
* OR2
* OR1
* ''cro'' gene
The lambda repressor is a self assembling dimer also known as the cI protein
Ci protein, short for Cubitus interruptus, is a zinc finger containing transcription factor involved in the Hedgehog signaling pathway. In the absence of a signal to the Hedgehog signaling pathway, the Ci protein is cleaved and destroyed in prote ...
. It binds DNA in the helix-turn-helix binding motif. It regulates the transcription of the cI protein and the Cro protein.
The life cycle of lambda phages is controlled by cI and Cro proteins. The lambda phage will remain in the lysogenic state if cI proteins predominate, but will be transformed into the lytic cycle if cro proteins predominate.
The cI dimer may bind to any of three operators, OR1, OR2, and OR3, in the order OR1 > OR2 > OR3.
Binding of a cI dimer to OR1 enhances binding of a second cI dimer to OR2, an effect called cooperativity. Thus, OR1 and OR2 are almost always simultaneously occupied by cI. However, this does not increase the affinity between cI and OR3, which will be occupied only when the cI concentration is high.
At high concentrations of cI, the dimers will also bind to operators OL1 and OL2 (which are over 2 kb downstream from the R operators). When cI dimers are bound to OL1, OL2, OR1, and OR2 a loop is induced in the DNA, allowing these dimers to bind together to form an octamer. This is a phenomenon called ''long-range cooperativity''. Upon formation of the octamer, cI dimers may cooperatively bind to OL3 and OR3, repressing transcription of cI. This ''autonegative'' regulation ensures a stable minimum concentration of the repressor molecule and, should SOS signals arise, allows for more efficient prophage induction.
* In the absence of cI proteins, the ''cro'' gene may be transcribed.
* In the presence of cI proteins, only the ''cI'' gene may be transcribed.
* At high concentration of cI, transcriptions of both genes are repressed.
Protein function overview
Lytic vs. lysogenic
 An important distinction here is that between the two decisions; lysogeny and lysis on infection, and continuing lysogeny or lysis from a prophage. The latter is determined solely by the activation of RecA in the SOS response of the cell, as detailed in the section on induction. The former will also be affected by this; a cell undergoing an SOS response will always be lysed, as no cI protein will be allowed to build up. However, the initial lytic/lysogenic decision on infection is also dependent on the cII and cIII proteins.
In cells with sufficient nutrients, protease activity is high, which breaks down cII. This leads to the lytic lifestyle. In cells with limited nutrients, protease activity is low, making cII stable. This leads to the lysogenic lifestyle. cIII appears to stabilize cII, both directly and by acting as a competitive inhibitor to the relevant proteases. This means that a cell "in trouble", i.e. lacking in nutrients and in a more dormant state, is more likely to lysogenise. This would be selected for because the phage can now lie dormant in the bacterium until it falls on better times, and so the phage can create more copies of itself with the additional resources available and with the more likely proximity of further infectable cells.
A full biophysical model for lambda's lysis-lysogeny decision remains to be developed. Computer modeling and simulation suggest that random processes during infection drive the selection of lysis or lysogeny within individual cells. However, recent experiments suggest that physical differences among cells, that exist prior to infection, predetermine whether a cell will lyse or become a lysogen.
An important distinction here is that between the two decisions; lysogeny and lysis on infection, and continuing lysogeny or lysis from a prophage. The latter is determined solely by the activation of RecA in the SOS response of the cell, as detailed in the section on induction. The former will also be affected by this; a cell undergoing an SOS response will always be lysed, as no cI protein will be allowed to build up. However, the initial lytic/lysogenic decision on infection is also dependent on the cII and cIII proteins.
In cells with sufficient nutrients, protease activity is high, which breaks down cII. This leads to the lytic lifestyle. In cells with limited nutrients, protease activity is low, making cII stable. This leads to the lysogenic lifestyle. cIII appears to stabilize cII, both directly and by acting as a competitive inhibitor to the relevant proteases. This means that a cell "in trouble", i.e. lacking in nutrients and in a more dormant state, is more likely to lysogenise. This would be selected for because the phage can now lie dormant in the bacterium until it falls on better times, and so the phage can create more copies of itself with the additional resources available and with the more likely proximity of further infectable cells.
A full biophysical model for lambda's lysis-lysogeny decision remains to be developed. Computer modeling and simulation suggest that random processes during infection drive the selection of lysis or lysogeny within individual cells. However, recent experiments suggest that physical differences among cells, that exist prior to infection, predetermine whether a cell will lyse or become a lysogen.
As a genetic tool
Lambda phage has been used heavily as a model organism, and has been a rich source for useful tools inmicrobial genetics
Microbial genetics is a subject area within microbiology and genetic engineering. Microbial genetics studies microorganisms for different purposes. The microorganisms that are observed are bacteria, and archaea. Some fungi and protozoa are also s ...
, and later in molecular genetics
Molecular genetics is a sub-field of biology that addresses how differences in the structures or expression of DNA molecules manifests as variation among organisms. Molecular genetics often applies an "investigative approach" to determine the ...
. Uses include its application as a vector for the cloning of recombinant DNA
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be fo ...
; the use of its site-specific recombinase (int) for the shuffling of cloned DNAs by the gateway method; and the application of its Red operon
In genetics, an operon is a functioning unit of DNA containing a cluster of genes under the control of a single promoter. The genes are transcribed together into an mRNA strand and either translated together in the cytoplasm, or undergo splic ...
, including the proteins Red alpha (also called 'exo'), beta and gamma in the DNA engineering method called recombineering. The 48 kb DNA fragment of lambda phage is not essential for productive infection and can be replaced by foreign DNA. Lambda phage will enter bacteria more easily than plasmids making it a useful vector that can destroy or can become part of the host's DNA. Lambda phage can be manipulated and used as an anti-cancer vaccine, nanoparticle, targeting human aspartyl (asparaginyl) β-hydroxylase (ASPH, HAAH). Lambda phage has also been of major importance in the study of specialized transduction.
See also
*Esther Lederberg
Esther Miriam Zimmer Lederberg (December 18, 1922 – November 11, 2006) was an American microbiologist and a pioneer of bacterial genetics. She discovered the bacterial virus λ and the bacterial fertility factor F, devised the first impl ...
* Lambda holin family
* Molecular weight size marker
A molecular-weight size marker, also referred to as a protein ladder, DNA ladder, or RNA ladder, is a set of standards that are used to identify the approximate size of a molecule run on a gel during electrophoresis, using the principle that ...
* Sankar Adhya
* Zygotic induction
Zygotic induction occurs when a bacterial cell carrying the silenced DNA of a bacterial virus in its chromosome transfers the viral DNA along with its own DNA to another bacterial cell lacking the virus, causing the recipient of the DNA to break o ...
* Corynebacteriophage
A corynebacteriophage (or just corynephage) is a DNA-containing bacteriophage specific for bacteria of genus ''Corynebacterium'' as its host.NCBICorynephages(list)
''Corynebacterium diphtheriae virus''NCBICorynebacterium diphtheriae virus/phage(s ...
s – Corynephages β (beta) and ω (omega) are (proposed) members of genus ''Lambdavirus''
References
Further reading
* James Watson, Tania Baker, Stephen Bell, Alexander Gann, Michael Levine, Richard Losick ''Molecular Biology of the Gene (International Edition)'' - 6th Edition *Mark Ptashne
Mark Ptashne (born June 5, 1940, in Chicago) is a molecular biologist. He is the Ludwig Chair of Molecular Biology at Memorial Sloan–Kettering Cancer Center in New York City.
Ptashne grew up in Chicago. He earned his undergraduate degree at Re ...
and Nancy Hopkins, "The Operators Controlled by the Lambda Phage Repressor", '' PNAS'', v.60, n.4, pp. 1282–1287 (1968).
* Barbara J. Meyer, Dennis G. Kleid, and Mark Ptashne, "Lambda Repressor Turns Off Transcription of Its Own Gene", ''PNAS'', v.72, n.12, pp. 4785–4789 (December 1975).
*
*
*
* Gottesman, M. and Weisberg, R.A. 2004 "Little lambda - who made thee?", ''Micro and Mol Biol Revs'', 68, 796-813 (available online a''Microbiology and Molecular Biology Reviews''
American Society for Microbiology
The American Society for Microbiology (ASM), originally the Society of American Bacteriologists, is a professional organization for scientists who study viruses, bacteria, fungi, algae, and protozoa as well as other aspects of microbiology. It wa ...
)
*
*
* Ptashne, M. "A Genetic Switch: Phage Lambda Revisited", 3rd edition 2003
*
* Snyder, L. and Champness, W. "Molecular Genetics of Bacteria", 3rd edition 2007 (Contains an informative and well illustrated overview of bacteriophage lambda)
* SplashoOnline overview of lambda
(illustrates genes active at all stages in lifecycle)
External links
* Life Cycle(illustrates infection and lytic/lysogenic pathways with some protein and transcription detail)
Time-lapse microscopy video
from MIT showing both lysis and lysogeny by phage lambda
Lambda Phage Life cycle
(basic visual demonstration of Lambda bacteriophage life cycle)
Lambda Phage genome in GenBank
Lambda Phage Reference Proteome from UniProt
Lambda Phage Protein Structures in NCBI
(3D display of protein structures for bacteriophage Lambda) {{DEFAULTSORT:Lambda Phage Genetics techniques Model organisms Siphoviridae