isophorone on:
[Wikipedia]
[Google]
[Amazon]
Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a
Isophorone history at Degussa
/ref>
solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
and as a precursor to polymers, it is produced on a large scale industrially.
Structure and reactivity
Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basichydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
affords the oxide.
Isophorone is degraded by attack of hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
s.
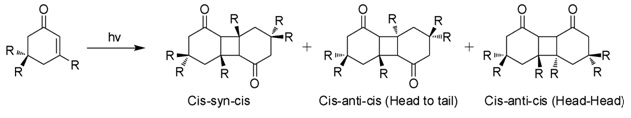
Photodimerization
When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium.
Natural Occurrence
Isophorone occurs naturally incranberries
Cranberries are a group of evergreen dwarf shrubs or trailing vines in the subgenus ''Oxycoccus'' of the genus ''Vaccinium''. In Britain, cranberry may refer to the native species ''Vaccinium oxycoccos'', while in North America, cranberry m ...
.
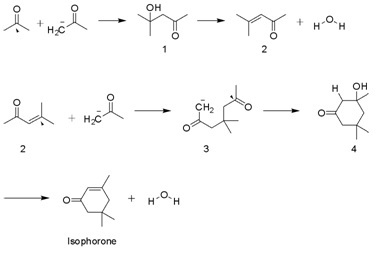
Synthesis
Isophorone is produced on a multi-thousand ton scale by the aldol condensation ofacetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
using KOH. Diacetone alcohol, mesityl oxide, and 3-hydroxy-3,5,5-trimethylcyclohexan-1-one are intermediates. A side product is beta-isophorone, where the C=C group is not conjugated with the ketone.

Applications
The partly hydrogenated derivative trimethylcyclohexanone is used in production ofpolycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
s. It condenses with phenol to give an analogue of bisphenol A
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial ...
. Polycarbonates produced by phosgenation of these two diols produces a polymer with improved thermal stability. Trimethyladipic acid
Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annuall ...
and 2,2,4-trimethylhexamethylenediamine are produced from trimethylcyclohexanone and trimethylcyclohexanol. They are used to make specialty polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made th ...
s. Hydrocyanation gives the nitrile followed by reductive amination gives isophorone diamine. This diamine is used to produce isophorone diisocyanate which has certain niche applications.
Full hydrogenation gives 3,3,5-Trimethylcyclohexanol, a precursor to both sunscreens and chemical weapons.
Safety
The LD50 value of isophorone in rats and rabbits by oral exposure is around the 2.00 g/kg. The safety aspects of isophorone have been subject to several studies.{{Cite journal, last=W. Morton Grant, Joel S. Schuman M.D, date=11 February 2016, title=Toxicology of the Eye: Effects on the Eyes and Visual System from Chemicals, Drugs, Metals and Minerals, Plants, Toxins, and Venoms; Also, Systemic Side Effects from Eye, journal=Med (2-Volume Set) 4th Edition, Page 863History
The use of isophorone as a solvent resulted from the search for ways to dispose of or recycleacetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
, which is a waste product of phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
synthesis by the Hock method./ref>
See also
* Phorone * beta-IsophoroneReferences