hydrophobic collapse on:
[Wikipedia]
[Google]
[Amazon]
Hydrophobic collapse is a proposed process for the production of the 3-D conformation adopted by

 The formation of a
The formation of a
polypeptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
and other molecules in polar solvents. The theory states that the nascent polypeptide forms initial secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
( ɑ-helices and β-strands) creating localized regions of predominantly hydrophobic residues. The polypeptide interacts with water, thus placing thermodynamic pressures on these regions which then aggregate or "collapse" into a tertiary conformation with a hydrophobic core. Incidentally, polar residues interact favourably with water, thus the solvent-facing surface of the peptide is usually composed of predominantly hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
regions.
Hydrophobic collapse may also reduce the affinity of conformationally flexible drugs to their protein targets by reducing the net hydrophobic contribution to binding by self association of different parts of the drug while in solution. Conversely rigid scaffolds (also called privileged structures) that resist hydrophobic collapse may enhance drug affinity.
Partial hydrophobic collapse is an experimentally accepted model for the folding kinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical ki ...
of many globular proteins, such as myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
, alpha-lactalbumin
Lactalbumin, alpha-, also known as LALBA, is a protein that in humans is encoded by the ''LALBA'' gene.
Overview
α-Lactalbumin is a protein that regulates the production of lactose in the milk of almost all mammalian species. In primates, alp ...
, barstar
Barstar is a small protein synthesized by the bacterium ''Bacillus amyloliquefaciens''. Its function is to inhibit the ribonuclease activity of its binding partner barnase
Barnase (a portmanteau of "BActerial" "RiboNucleASE") is a bacterial pr ...
, and staphylococcal
''Staphylococcus'' is a genus of Gram-positive bacteria in the family Staphylococcaceae from the order Bacillales. Under the microscope, they appear spherical (cocci), and form in grape-like clusters. ''Staphylococcus'' species are facultative ...
nuclease
A nuclease (also archaically known as nucleodepolymerase or polynucleotidase) is an enzyme capable of cleaving the phosphodiester bonds between nucleotides of nucleic acids. Nucleases variously effect single and double stranded breaks in their ta ...
. However, because experimental evidence of early folding events is difficult to obtain, hydrophobic collapse is often studied ''in silico'' via molecular dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the ...
and Monte Carlo
Monte Carlo (; ; french: Monte-Carlo , or colloquially ''Monte-Carl'' ; lij, Munte Carlu ; ) is officially an administrative area of the Principality of Monaco, specifically the ward of Monte Carlo/Spélugues, where the Monte Carlo Casino is ...
simulations of the folding process. Globular proteins that are thought to fold by hydrophobic collapse are particularly amenable to complementary computational and experimental study using phi value analysis Phi value analysis,
\phi
analysis, or \phi-value analysis is an experimental protein engineering technique for studying the structure of the folding transition state of small protein domains that fold in a two-state manner. The structure of the f ...
.
Biological significance
Correctprotein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
is integral to proper functionality within biological system
A biological system is a complex network which connects several biologically relevant entities. Biological organization spans several scales and are determined based different structures depending on what the system is. Examples of biological syst ...
s. Hydrophobic collapse is one of the main events necessary for reaching a protein's stable and functional conformation. Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s perform extremely specific functions which are dependent on their structure. Proteins that do not fold correctly are nonfunctional and contribute nothing to a biological system.
Hydrophobic aggregation can also occur between unrelated polypeptides. If two locally hydrophobic regions of two unrelated structures are left near each other in aqueous solution, aggregation will occur. In this case, this can have drastic effects on the health of the organism
In biology, an organism () is any living system that functions as an individual entity. All organisms are composed of cells (cell theory). Organisms are classified by taxonomy into groups such as multicellular animals, plants, and ...
. The formation of amyloid fibrils
Amyloids are aggregates of proteins characterised by a fibrillar morphology of 7–13 nm in diameter, a beta sheet (β-sheet) secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human ...
, insoluble aggregates of hydrophobic protein can lead to a myriad of diseases including Parkinson's
Parkinson's disease (PD), or simply Parkinson's, is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms becom ...
and Alzheimer's disease
Alzheimer's disease (AD) is a neurodegeneration, neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in short-term me ...
.Energetics
The driving force behind protein folding is not well understood, hydrophobic collapse is atheory
A theory is a rational type of abstract thinking about a phenomenon, or the results of such thinking. The process of contemplative and rational thinking is often associated with such processes as observational study or research. Theories may be s ...
, one of many, that is thought to influence how a nascent
Nascent may refer to:
* '' Nascent'', a 2005 Australian dance film with choreography by Garry Stewart
* '' Nascent (film)'', a 2016 Central African short documentary film by Lindsay Branham and Jon Kasbe
See also
*
*
* Nascent hydrogen, disc ...
polypeptide will fold into its native state. Hydrophobic collapse can be visualized as part of the folding funnel
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells. ...
model which leads a protein to its lowest kinetically accessible energy state. In this model, we do not consider the interactions of the peptide backbone as this maintains its stability in non-polar and polar environments as long as there is sufficient hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing within the backbone, thus we will only consider the thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of ther ...
contributions of the side chains to protein stability.
When placed in a polar solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
, polar side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a l ...
s can form weak intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
interactions with the solvent, specifically hydrogen bonding. The solvent is able to maintain hydrogen bonding with itself as well as the polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
. This maintains the stability of the structure within localized segments of the protein. However, non-polar side chains are unable to participate in hydrogen bonding interactions. The inability of the solvent to interact with these side chains leads to a decrease in entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
of the system. The solvent can interact with itself, however the portion of the molecule in proximity to the non-polar side chain is unable to form any significant interactions, thus the dissociative degrees of freedom available to the molecule decreases and entropy decreases. By aggregating the hydrophobic regions, the solvent can reduce the surface area exposed to non-polar side chains, thus reduce localized areas of decreased entropy. While the entropy of the polypeptide has decreased as it enters a more ordered state, the overall entropy of the system increases, contributing to the thermodynamic favourability of a folded polypeptide.
As can be seen in the folding funnel
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds to its free energy minimum under the solution conditions usually encountered in cells. ...
diagram, the polypeptide is at its highest energy state
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The te ...
when unfolded in aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
. As it forms localized folding intermediates, or molten globules, the energy of the system decreases. The polypeptide will continue folding into lower energy states as long as these conformations are kinetically accessible. In this case, a native conformation does not have to be at the lowest energy trough of the diagram as shown, it must simply exist in its natural and kinetically accessible conformation in biological systems.

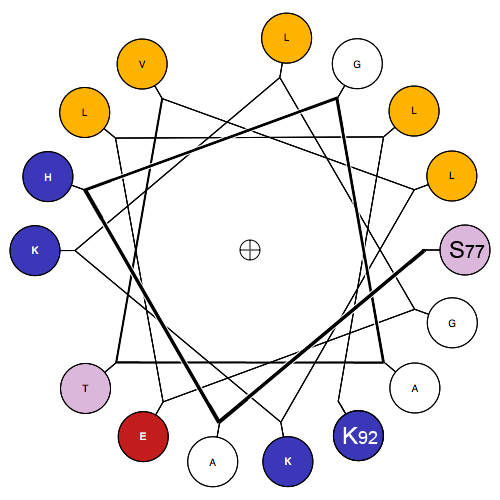
Surface structures
 The formation of a
The formation of a hydrophobic core
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar ...
requires the surface structures of this aggregate to maintain contact with both the polar solvent as well as the internal structures. In order to do this, these surface structures usually contain amphipathic
An amphiphile (from the Greek αμφις amphis, both, and φιλíα philia, love, friendship), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'') properties. Such a compoun ...
properties. A surface exposed alpha helix may have nonpolar residues in an N+3, N+4 position, allowing the alpha-helix to express nonpolar properties on one side when split longitudinally along the axis. Note, in the diagram, the presence of non-polar(gold) amino acids along one side of the helix when viewed through the longitudinal axis, as well as charged/polar amino acids along the other face. This provides this structure with longitudinal amphipathic properties necessary for hydrophobic aggregation along the non-polar side. Similarly, beta strands can also adopt this property with simple alternation of polar and nonpolar residues. Every N+1 side chain will occupy space on the opposite side of the beta strand.
References
{{reflist, 2 Protein structure