Hydrogen Chloride on:
[Wikipedia]
[Google]
[Amazon]
The
 Hydrogen chloride is a
Hydrogen chloride is a

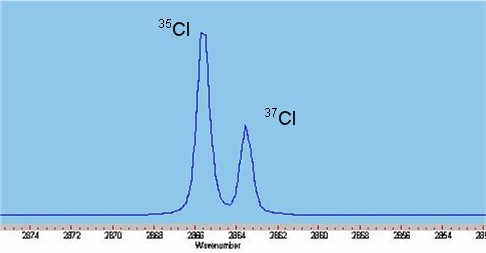 The infrared spectrum of gaseous hydrogen chloride, shown on the left, consists of a number of sharp absorption lines grouped around 2886 cm−1 (wavelength ~3.47 µm). At room temperature, almost all molecules are in the ground vibrational state ''v'' = 0. Including anharmonicity the vibrational energy can be written as.
::
To promote an HCl molecule from the ''v'' = 0 to the ''v'' = 1 state, we would expect to see an infrared absorption about ''ν''o = ''ν''e + 2''x''e''ν''e = 2880 cm−1. However, this absorption corresponding to the Q-branch is not observed due to it being forbidden by symmetry. Instead, two sets of signals (P- and R-branches) are seen owing to a simultaneous change in the rotational state of the molecules. Because of quantum mechanical selection rules, only certain rotational transitions are permitted. The states are characterized by the rotational quantum number ''J'' = 0, 1, 2, 3, ... selection rules state that Δ''J'' is only able to take values of ±1.
::
The value of the rotational constant ''B'' is much smaller than the vibrational one ''ν''o, such that a much smaller amount of energy is required to rotate the molecule; for a typical molecule, this lies within the microwave region. However, the vibrational energy of HCl molecule places its absorptions within the infrared region, allowing a spectrum showing the rovibrational transitions of this molecule to be easily collected using an
The infrared spectrum of gaseous hydrogen chloride, shown on the left, consists of a number of sharp absorption lines grouped around 2886 cm−1 (wavelength ~3.47 µm). At room temperature, almost all molecules are in the ground vibrational state ''v'' = 0. Including anharmonicity the vibrational energy can be written as.
::
To promote an HCl molecule from the ''v'' = 0 to the ''v'' = 1 state, we would expect to see an infrared absorption about ''ν''o = ''ν''e + 2''x''e''ν''e = 2880 cm−1. However, this absorption corresponding to the Q-branch is not observed due to it being forbidden by symmetry. Instead, two sets of signals (P- and R-branches) are seen owing to a simultaneous change in the rotational state of the molecules. Because of quantum mechanical selection rules, only certain rotational transitions are permitted. The states are characterized by the rotational quantum number ''J'' = 0, 1, 2, 3, ... selection rules state that Δ''J'' is only able to take values of ±1.
::
The value of the rotational constant ''B'' is much smaller than the vibrational one ''ν''o, such that a much smaller amount of energy is required to rotate the molecule; for a typical molecule, this lies within the microwave region. However, the vibrational energy of HCl molecule places its absorptions within the infrared region, allowing a spectrum showing the rovibrational transitions of this molecule to be easily collected using an
 This "acetylene process" has been replaced by a process which adds to the double bond of ethylene instead, and subsequent elimination produces HCl instead, as well as chloroprene.
This "acetylene process" has been replaced by a process which adds to the double bond of ethylene instead, and subsequent elimination produces HCl instead, as well as chloroprene.
compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
hydrogen chloride has the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
and as such is a hydrogen halide
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, or astatine. ...
. At room temperature, it is a colourless gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
, which forms white fumes of hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
upon contact with atmospheric water vapor
(99.9839 °C)
, -
, Boiling point
,
, -
, specific gas constant
, 461.5 J/( kg·K)
, -
, Heat of vaporization
, 2.27 MJ/kg
, -
, Heat capacity
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gaseous p ...
. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl.
Reactions
 Hydrogen chloride is a
Hydrogen chloride is a diatomic molecule
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. O ...
, consisting of a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
H and a chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge A partial charge is a non-integer charge value when measured in elementary charge units. Partial charge is more commonly called net atomic charge. It is represented by the Greek lowercase letter 𝛿, namely 𝛿− or 𝛿+.
Partial charges are c ...
(δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
(and in other polar solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s).
Upon contact, and HCl combine to form hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid i ...
cations and chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s through a reversible chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
:
:
The resulting solution is called hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
and is a strong acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions ...
. The acid dissociation or ionization constant, ''K''a, is large, which means HCl dissociates or ionizes practically completely in water. Even in the absence of water, hydrogen chloride can still act as an acid. For example, hydrogen chloride can dissolve in certain other solvents such as methanol:
:
Hydrogen chloride can protonate molecules or ions and can also serve as an acid-catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
for chemical reactions where anhydrous (water-free) conditions are desired.
Because of its acidic nature, hydrogen chloride is a corrosive substance, particularly in the presence of moisture.
Structure and properties
Frozen HCl undergoes phase transition at 98.4 K. X-ray powder diffraction of the frozen material shows that the material changes from an orthorhombic structure to a cubic one during this transition. In both structures the chlorine atoms are in a face-centered array. However, the hydrogen atoms could not be located. Analysis of spectroscopic and dielectric data, and determination of the structure of DCl (deuterium chloride) indicates that HCl forms zigzag chains in the solid, as does HF (see figure on right).
infrared spectrometer
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functiona ...
with a gas cell. The latter can even be made of quartz as the HCl absorption lies in a window of transparency for this material.
Naturally abundant chlorine consists of two isotopes, 35Cl and 37Cl, in a ratio of approximately 3:1. While the spring constants are nearly identical, the disparate reduced mass
In physics, the reduced mass is the "effective" inertial mass appearing in the two-body problem of Newtonian mechanics. It is a quantity which allows the two-body problem to be solved as if it were a one-body problem. Note, however, that the mass ...
es of H35Cl and H37Cl cause measurable differences in the rotational energy, thus doublets are observed on close inspection of each absorption line, weighted in the same ratio of 3:1.
Production
Most hydrogen chloride produced on an industrial scale is used forhydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
production.
Historical routes
In the 17th century,Johann Rudolf Glauber
Johann Rudolf Glauber (10 March 1604 – 16 March 1670) was a German-Dutch alchemist and chemist. Some historians of science have described him as one of the first chemical engineers. His discovery of sodium sulfate in 1625 led to the compou ...
from Karlstadt am Main, Germany used sodium chloride salt and sulfuric acid for the preparation of sodium sulfate in the Mannheim process, releasing hydrogen chloride. Joseph Priestley of Leeds, England
Leeds () is a city and the administrative centre of the City of Leeds district in West Yorkshire, England. It is built around the River Aire and is in the eastern foothills of the Pennines. It is also the third-largest settlement (by populat ...
prepared pure hydrogen chloride in 1772, and by 1808 Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
of Penzance, England had proved that the chemical composition included hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
.
Direct synthesis
Hydrogen chloride is produced by combiningchlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
:
:
As the reaction is exothermic, the installation is called an HCl oven or HCl burner. The resulting hydrogen chloride gas is absorbed in deionized water, resulting in chemically pure hydrochloric acid. This reaction can give a very pure product, e.g. for use in the food industry.
The reaction can also be triggered by blue light.
Organic synthesis
The industrial production of hydrogen chloride is often integrated with the formation ofchlorinated
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polyme ...
and fluorinated organic compounds, e.g., Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemo ...
, Freon
Freon ( ) is a registered trademark of the Chemours Company and generic descriptor for a number of halocarbon products. They are stable, nonflammable, low toxicity gases or liquids which have generally been used as refrigerants and as aerosol prope ...
, and other CFCs, as well as chloroacetic acid
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds a ...
and PVC. Often this production of hydrochloric acid is integrated with captive use of it on-site. In the chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s, hydrogen atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom recombines with the spare atom from the chlorine molecule, forming hydrogen chloride. Fluorination is a subsequent chlorine-replacement reaction, producing again hydrogen chloride:
:
:RCl + HF → RF + HCl
The resulting hydrogen chloride is either reused directly or absorbed in water, resulting in hydrochloric acid of technical or industrial grade.
Laboratory methods
Small amounts of hydrogen chloride for laboratory use can be generated in an ''HCl generator'' by dehydrating hydrochloric acid with either sulfuric acid or anhydrouscalcium chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Ca ...
. Alternatively, HCl can be generated by the reaction of sulfuric acid with sodium chloride:
:
This reaction occurs at room temperature. Provided there is NaCl remaining in the generator and it is heated above 200 °C, the reaction proceeds further:
:
For such generators to function, the reagents should be dry.
Hydrogen chloride can also be prepared by the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
of certain reactive chloride compounds such as phosphorus chlorides, thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year bein ...
(), and acyl chlorides. For example, cold water can be gradually dripped onto phosphorus pentachloride () to give HCl:
:
Applications
Most hydrogen chloride is used in the production of hydrochloric acid. It is also used in the production ofvinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
and many alkyl chlorides. Trichlorosilane
Trichlorosilane is an inorganic compound with the formula HCl3Si. It is a colourless, volatile liquid. Purified trichlorosilane is the principal precursor to ultrapure silicon in the semiconductor industry. In water, it rapidly decomposes to pr ...
is produced using HCl:
:
History
Around 900, the authors of the Arabic writings attributed toJabir ibn Hayyan
Abū Mūsā Jābir ibn Ḥayyān (Arabic: , variously called al-Ṣūfī, al-Azdī, al-Kūfī, or al-Ṭūsī), died 806−816, is the purported author of an enormous number and variety of works in Arabic, often called the Jabirian corpus. The ...
(Latin: Geber) and the Persian physician and alchemist Abu Bakr al-Razi
Abū Bakr al-Rāzī (full name: ar, أبو بکر محمد بن زکریاء الرازي, translit=Abū Bakr Muḥammad ibn Zakariyyāʾ al-Rāzī, label=none), () rather than ar, زکریاء, label=none (), as for example in , or in . In m ...
(c. 865–925, Latin: Rhazes) were experimenting with sal ammoniac ( ammonium chloride), which when it was distilled together with vitriol
Vitriol is the general chemical name encompassing a class of chemical compound comprising sulfates of certain metalsoriginally, iron or copper. Those mineral substances were distinguished by their color, such as green vitriol for hydrated iron( ...
(hydrated sulfates
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many a ...
of various metals) produced hydrogen chloride. It is possible that in one of his experiments, al-Razi stumbled upon a primitive method to produce hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
. However, it appears that in most of these early experiments with chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively c ...
, the gaseous products were discarded, and hydrogen chloride may have been produced many times before it was discovered that it can be put to chemical use.
One of the first such uses was the synthesis of mercury(II) chloride (corrosive sublimate), whose production from the heating of mercury either with alum and ammonium chloride or with vitriol and sodium chloride was first described in the ''De aluminibus et salibus'' ("On Alums and Salts"), an eleventh- or twelfth century Arabic text falsely attributed to Abu Bakr al-Razi and translated into Latin by Gerard of Cremona
Gerard of Cremona (Latin: ''Gerardus Cremonensis''; c. 1114 – 1187) was an Italian translator of scientific books from Arabic into Latin. He worked in Toledo, Kingdom of Castile and obtained the Arabic books in the libraries at Toledo. Some of ...
(1144–1187).
Another important development was the discovery by pseudo-Geber
Pseudo-Geber (or "Latin pseudo-Geber") is the presumed author or group of authors responsible for a corpus of pseudepigraphic alchemical writings dating to the late 13th and early 14th centuries. These writings were falsely attributed to Jabir ...
(in the ''De inventione veritatis'', "On the Discovery of Truth", after c. 1300) that by adding ammonium chloride to nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
, a strong solvent capable of dissolving gold (i.e., '' aqua regia'') could be produced.
After the discovery in the late sixteenth century of the process by which unmixed hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
can be prepared, it was recognized that this new acid (then known as ''spirit of salt'' or ''acidum salis'') released vaporous hydrogen chloride, which was called ''marine acid air''. In the 17th century, Johann Rudolf Glauber
Johann Rudolf Glauber (10 March 1604 – 16 March 1670) was a German-Dutch alchemist and chemist. Some historians of science have described him as one of the first chemical engineers. His discovery of sodium sulfate in 1625 led to the compou ...
used salt ( sodium chloride) and sulfuric acid for the preparation of sodium sulfate, releasing hydrogen chloride gas (see production, above). In 1772, Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hyd ...
also reported this reaction and is sometimes credited with its discovery. Joseph Priestley prepared hydrogen chloride in 1772, and in 1810 Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
established that it is composed of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
and chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
.
During the Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going f ...
, demand for alkaline substances such as soda ash
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
increased, and Nicolas Leblanc
Nicolas Leblanc (December 6, 1742 – January 16, 1806) was a French chemist and surgeon who discovered how to manufacture soda ash from common salt.
Earlier days
Leblanc was born in Ivoy le Pré, Cher, France on 6 December 1742. His fathe ...
developed a new industrial-scale process for producing the soda ash. In the Leblanc process, salt was converted to soda ash, using sulfuric acid, limestone, and coal, giving hydrogen chloride as by-product. Initially, this gas was vented to air, but the Alkali Act of 1863 prohibited such release, so then soda ash producers absorbed the HCl waste gas in water, producing hydrochloric acid on an industrial scale. Later, the Hargreaves process was developed, which is similar to the Leblanc process except sulfur dioxide, water, and air are used instead of sulfuric acid in a reaction which is exothermic overall. In the early 20th century the Leblanc process was effectively replaced by the Solvay process
The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s ...
, which did not produce HCl. However, hydrogen chloride production continued as a step in hydrochloric acid production.
Historical uses of hydrogen chloride in the 20th century include hydrochlorinations of alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
in producing the chlorinated monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s chloroprene
Chloroprene is the common name for 2-chlorobuta-1,3-diene (IUPAC name) with the chemical formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene ...
and vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
, which are subsequently polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ized to make polychloroprene ( Neoprene) and polyvinyl chloride (PVC), respectively. In the production of vinyl chloride, acetylene () is hydrochlorinated by adding the HCl across the triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
of the molecule, turning the triple into a double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
, yielding vinyl chloride.
The "acetylene process", used until the 1960s for making chloroprene
Chloroprene is the common name for 2-chlorobuta-1,3-diene (IUPAC name) with the chemical formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene ...
, starts out by joining two acetylene molecules, and then adds HCl to the joined intermediate across the triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
to convert it to chloroprene as shown here:
:Safety
Hydrogen chloride forms corrosivehydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
on contact with water found in body tissue. Inhalation of the fumes can cause cough
A cough is a sudden expulsion of air through the large breathing passages that can help clear them of fluids, irritants, foreign particles and microbes. As a protective reflex, coughing can be repetitive with the cough reflex following three ph ...
ing, choking
Choking, also known as foreign body airway obstruction (FBAO), is a phenomenon that occurs when breathing is impeded by a blockage inside of the respiratory tract. An obstruction that prevents oxygen from entering the lungs results in oxygen dep ...
, inflammation
Inflammation (from la, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants, and is a protective response involving immune cells, blood vessels, and molec ...
of the nose, throat, and upper respiratory tract, and in severe cases, pulmonary edema
Pulmonary edema, also known as pulmonary congestion, is excessive liquid accumulation in the tissue and air spaces (usually alveoli) of the lungs. It leads to impaired gas exchange and may cause hypoxemia and respiratory failure. It is due t ...
, circulatory system
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, tha ...
failure, and death. Skin contact can cause redness, pain, and severe chemical burn
A chemical burn occurs when living tissue is exposed to a corrosive substance (such as a strong acid, base or oxidizer) or a cytotoxic agent (such as mustard gas, lewisite or arsine). Chemical burns follow standard burn classification and may c ...
s. Hydrogen chloride may cause severe burns to the eye and permanent eye damage.
The U.S. Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the C ...
have established occupational exposure limits for hydrogen chloride at a ceiling of 5 ppm (7 mg/m3), and compiled extensive information on hydrogen chloride workplace safety concerns.
See also
* Gastric acid, hydrochloric acid secreted into thestomach
The stomach is a muscular, hollow organ in the gastrointestinal tract of humans and many other animals, including several invertebrates. The stomach has a dilated structure and functions as a vital organ in the digestive system. The stomach i ...
to aid digestion
Digestion is the breakdown of large insoluble food molecules into small water-soluble food molecules so that they can be absorbed into the watery blood plasma. In certain organisms, these smaller substances are absorbed through the small intest ...
of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s
* Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
, salts of hydrogen chloride
* Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative n ...
, organic salts of hydrochloric acid
* Hydrochlorination
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.
:
If the two carbon atoms at the double bond are linked to a different n ...
, addition reaction with alkenes
References
External links
* * Thames & Kosmos Chem C2000 Experiment Manual {{Authority control Chlorides Halogen-containing natural products Hazardous air pollutants Hydrogen compounds Industrial gases Nonmetal halides Articles containing video clips