hexamethylbenzene on:
[Wikipedia]
[Google]
[Amazon]
Hexamethylbenzene, also known as mellitene, is a
 Treatment of hexamethylbenzene with a superelectrophilic mixture of methyl chloride and aluminum trichloride (a source of Meδ⊕Cl---δ⊖AlCl3) gives heptamethylbenzenium cation, one of the first carbocations to be directly observed.
Treatment of hexamethylbenzene with a superelectrophilic mixture of methyl chloride and aluminum trichloride (a source of Meδ⊕Cl---δ⊖AlCl3) gives heptamethylbenzenium cation, one of the first carbocations to be directly observed.

 The mechanisms of such surface-mediated reactions have been investigated, with an eye to achieving greater control over the outcome of the reaction, especially in search of selective and controlled '' ortho''-methylation. Both anisole and pentamethylbenzene have been reported as intermediates in the process.
The mechanisms of such surface-mediated reactions have been investigated, with an eye to achieving greater control over the outcome of the reaction, especially in search of selective and controlled '' ortho''-methylation. Both anisole and pentamethylbenzene have been reported as intermediates in the process. 
 It has also been used as a solvent for 3He-NMR spectroscopy.
Just as with benzene itself, the electron-rich aromatic system in hexamethylbenzene allows it to act as a
It has also been used as a solvent for 3He-NMR spectroscopy.
Just as with benzene itself, the electron-rich aromatic system in hexamethylbenzene allows it to act as a  In the field of organoruthenium chemistry, the redox interconversion of the analogous two-electron reduction of the dication and its neutral product occurs at −1.02 V in
In the field of organoruthenium chemistry, the redox interconversion of the analogous two-electron reduction of the dication and its neutral product occurs at −1.02 V in
 The isolation of an ion with composition was first reported from investigations of hexamethyl Dewar benzene in the 1960s; a pyramidal structure was suggested based on
The isolation of an ion with composition was first reported from investigations of hexamethyl Dewar benzene in the 1960s; a pyramidal structure was suggested based on 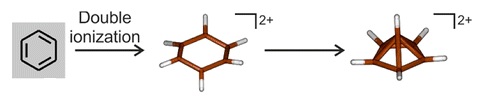
 Two-electron oxidation of hexamethylbenzene would be expected to result in a near-identical rearrangement to a pyramidal carbocation, but attempts to synthesise it in bulk by this method have been unsuccessful. However, a modification of the Hogeveen approach was reported in 2016, along with a high-quality crystal structure determination of . The pyramidal core is about 1.18
Two-electron oxidation of hexamethylbenzene would be expected to result in a near-identical rearrangement to a pyramidal carbocation, but attempts to synthesise it in bulk by this method have been unsuccessful. However, a modification of the Hogeveen approach was reported in 2016, along with a high-quality crystal structure determination of . The pyramidal core is about 1.18  Though indirect spectroscopic evidence and theoretical calculations previously pointed to their existence, the isolation and structural determination of a species with a hexacoordinate carbon bound only to other carbon atoms is unprecedented, and has attracted comment in ''
Though indirect spectroscopic evidence and theoretical calculations previously pointed to their existence, the isolation and structural determination of a species with a hexacoordinate carbon bound only to other carbon atoms is unprecedented, and has attracted comment in ''
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
with the molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
C12H18 and the condensed structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bondi ...
C6(CH3)6. It is an aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
and a derivative
In mathematics, the derivative of a function of a real variable measures the sensitivity to change of the function value (output value) with respect to a change in its argument (input value). Derivatives are a fundamental tool of calculus. ...
of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
, where benzene's six hydrogen atoms have each been replaced by a methyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ma ...
. In 1929 Kathleen Lonsdale reported the crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
and for understanding aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
.
The compound can be prepared by reacting phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
with methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
at elevated temperatures over a suitable solid catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
such as alumina. The mechanism of the process has been studied extensively, with several intermediates having been identified. Alkyne trimerisation
In organic chemistry, an alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applica ...
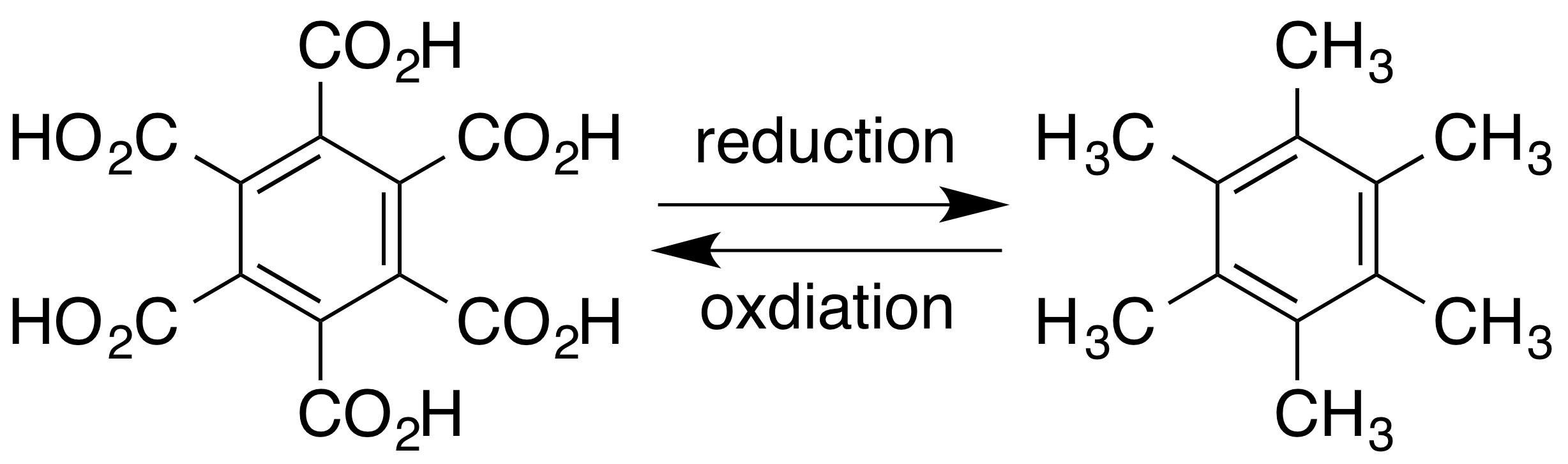
of dimethylacetylene also yields hexamethylbenzene in the presence of a suitable catalyst. Hexamethylbenzene can be oxidised to mellitic acid
Mellitic acid, also called graphitic acid or benzenehexacarboxylic acid, is an acid first discovered in 1799 by Martin Heinrich Klaproth in the mineral mellite (honeystone), which is the aluminium salt of the acid. It crystallizes in fine silky ne ...
, which is found in nature as its aluminium salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
in the rare mineral mellite
Mellite, also called honeystone, is an unusual mineral being also an organic chemical. It is chemically identified as an aluminium salt of mellitic acid, and specifically as aluminium benzene hexacarboxylate hydrate, with the chemical formula Al ...
. Hexamethylbenzene can be used as a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
in organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
s. An example from organoruthenium chemistry shows structural change in the ligand associated with changes in the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of the metal centre, though the same change is not observed in the analogous
Analogy (from Greek ''analogia'', "proportion", from ''ana-'' "upon, according to" lso "against", "anew"+ ''logos'' "ratio" lso "word, speech, reckoning" is a cognitive process of transferring information or meaning from a particular subject ...
organoiron system.
In 2016 the crystal structure of the hexamethylbenzene dication was reported in ''Angewandte Chemie International Edition
''Angewandte Chemie'' (, meaning "Applied Chemistry") is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society (Gesellschaft Deutscher Chemiker). Publishing formats include feature-leng ...
'', showing a pyramidal structure in which a single carbon atom has a bonding interaction with six other carbon atoms. This structure was "unprecedented", as the usual maximum valence of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
is four, and it attracted attention from ''New Scientist
''New Scientist'' is a magazine covering all aspects of science and technology. Based in London, it publishes weekly English-language editions in the United Kingdom, the United States and Australia. An editorially separate organisation publish ...
'', ''Chemical & Engineering News
''Chemical & Engineering News'' (''C&EN'') is a weekly news magazine published by the American Chemical Society, providing professional and technical news and analysis in the fields of chemistry and chemical engineering.Science News
''Science News (SN)'' is an American bi-weekly magazine devoted to articles about new scientific and technical developments, typically gleaned from recent scientific and technical journals.
History
''Science News'' has been published since ...
''. The structure does not violate the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rul ...
since the carbon–carbon bonds formed are not two-electron bonds, and is pedagogically
Pedagogy (), most commonly understood as the approach to teaching, is the theory and practice of learning, and how this process influences, and is influenced by, the social, political and psychological development of learners. Pedagogy, taken a ...
valuable for illustrating that a carbon atom "can irectly bondwith more than four atoms". Steven Bachrach Steven M. Bachrach is an organic chemist who took up the position of Dean of Science at Monmouth University in 2016. Bachrach had previously been the Dr D. R. Semmes Distinguished Professor of Chemistry at Trinity University in San Antonio, Texas. ...
has demonstrated that the compound is hypercoordinated but not hypervalent
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pe ...
, and also explained its aromaticity. The idea of describing the bonding in species like this through the lens of organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
was proposed in 1975, soon after was first observed.
Nomenclature and properties
According to the ''Blue Book
A blue book or bluebook is an almanac, buyer's guide or other compilation of statistics and information. The term dates back to the 15th century, when large blue velvet-covered books were used for record-keeping by the Parliament of England. The ...
'', this chemical can be systematically named as 1,2,3,4,5,6-hexamethylbenzene. The locants (the numbers in front of the name) are superfluous, however, as the name hexamethylbenzene uniquely identifies a single substance and thus is the formal IUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
for the compound. It is an aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
, with six π electrons (satisfying Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was ...
) delocalised over a cyclic planar system; each of the six ring carbon atoms is sp2 hybridised and displays trigonal planar
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands ...
geometry
Geometry (; ) is, with arithmetic, one of the oldest branches of mathematics. It is concerned with properties of space such as the distance, shape, size, and relative position of figures. A mathematician who works in the field of geometry is c ...
, while each methyl carbon is tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ...
with sp3 hybridisation, consistent with the empirical description of its structure. Solid hexamethylbenzene occurs as colourless to white crystalline orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with ...
prisms or needles with a melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
of 165–166 °C, a boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding env ...
of 268 °C, and a density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematicall ...
of 1.0630 g cm−3. It is insoluble in water, but soluble in organic solvents including benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
.
Hexamethylbenzene is sometimes called mellitene, a name derived from mellite
Mellite, also called honeystone, is an unusual mineral being also an organic chemical. It is chemically identified as an aluminium salt of mellitic acid, and specifically as aluminium benzene hexacarboxylate hydrate, with the chemical formula Al ...
, a rare honey-coloured mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
( ''meli'' (GEN
Gen may refer to:
* ''Gen'' (film), 2006 Turkish horror film directed by Togan Gökbakar
* Gen (Street Fighter), a video game character from the ''Street Fighter'' series
* Gen Fu, a video game character from the ''Dead or Alive'' series
* Gen l ...
''melitos'') is the Greek word for honey.) Mellite is composed of a hydrated
Drinking is the act of ingesting water or other liquids into the body through the mouth, proboscis, or elsewhere. Humans drink by swallowing, completed by peristalsis in the esophagus. The physiological processes of drinking vary widely among o ...
aluminium salt of benzenehexacarboxylic acid (mellitic acid), with formula . Mellitic acid itself can be derived from the mineral, and subsequent reduction yields mellitene. Conversely, mellitene can be oxidised
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
to form mellitic acid:
 Treatment of hexamethylbenzene with a superelectrophilic mixture of methyl chloride and aluminum trichloride (a source of Meδ⊕Cl---δ⊖AlCl3) gives heptamethylbenzenium cation, one of the first carbocations to be directly observed.
Treatment of hexamethylbenzene with a superelectrophilic mixture of methyl chloride and aluminum trichloride (a source of Meδ⊕Cl---δ⊖AlCl3) gives heptamethylbenzenium cation, one of the first carbocations to be directly observed.

Structure
In 1927 Kathleen Lonsdale determined the solid structure of hexamethylbenzene from crystals provided byChristopher Kelk Ingold
Sir Christopher Kelk Ingold (28 October 1893 – 8 December 1970) was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was resp ...
. Her X-ray diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
analysis was published in ''Nature
Nature, in the broadest sense, is the physical world or universe. "Nature" can refer to the phenomena of the physical world, and also to life in general. The study of nature is a large, if not the only, part of science. Although humans are ...
'' and was subsequently described as "remarkable ... for that early date". Lonsdale described the work in her book ''Crystals and X-Rays'', explaining that she recognised that, though the unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
was triclinic
180px, Triclinic (a ≠ b ≠ c and α ≠ β ≠ γ )
In crystallography, the triclinic (or anorthic) crystal system is one of the 7 crystal systems. A crystal system is described by three basis vectors. In the triclinic system, the crystal i ...
, the diffraction pattern
Diffraction is defined as the interference or bending of waves around the corners of an obstacle or through an aperture into the region of geometrical shadow of the obstacle/aperture. The diffracting object or aperture effectively becomes a ...
had pseudo-hexagonal symmetry that allowed the structural possibilities to be restricted sufficiently for a trial-and-error approach to produce a model. This work definitively showed that hexamethylbenzene is flat and that the carbon-to-carbon distances within the ring are the same, providing crucial evidence in understanding the nature of aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
.
Preparation
In 1880 Joseph Achille Le Bel and William H. Greene reported what has been described as an "extraordinary"zinc chloride
Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic ...
-catalysed
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
one-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy sepa ...
of hexamethylbenzene from methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
. At the catalyst's melting point (283 °C), the reaction has a Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature an ...
(ΔG) of −1090 kJ mol−1 and can be idealised as:
:15 → + 3 + 15
Le Bel and Greene rationalised the process as involving aromatisation by condensation of methylene units, formed by dehydration of methanol molecules, followed by complete Friedel–Crafts methylation of the resulting benzene ring with chloromethane
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ...
generated ''in situ''. The major products were a mixture of saturated hydrocarbon
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whic ...
s, with hexamethylbenzene as a minor product. Hexamethylbenzene is also produced as a minor product in the Friedel–Crafts alkylation synthesis of durene from ''p''-xylene, and can be produced by alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
in good yield from durene or pentamethylbenzene.
Hexamethylbenzene is typically prepared in the gas phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetiza ...
at elevated temperatures over solid catalysts. An early approach to preparing hexamethylbenzene involved reacting a mixture of acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
and methanol vapours over an alumina catalyst at 400 °C. Combining phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
s with methanol over alumina in a dry carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
atmosphere at 410–440 °C also produces hexamethylbenzene, though as part of a complex mixture of anisole
Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound i ...
(methoxybenzene), cresol
Cresols (also hydroxytoluene or cresylic acid) are a group of aromatic organic compounds. They are widely-occurring phenols (sometimes called ''phenolics'') which may be either natural or manufactured. They are also categorized as methylphenol ...
s (methylphenols), and other methylated phenols. An ''Organic Syntheses'' preparation, using methanol and phenol with an alumina catalyst at 530 °C, gives approximately a 66% yield, though synthesis under different conditions has also been reported.
 The mechanisms of such surface-mediated reactions have been investigated, with an eye to achieving greater control over the outcome of the reaction, especially in search of selective and controlled '' ortho''-methylation. Both anisole and pentamethylbenzene have been reported as intermediates in the process.
The mechanisms of such surface-mediated reactions have been investigated, with an eye to achieving greater control over the outcome of the reaction, especially in search of selective and controlled '' ortho''-methylation. Both anisole and pentamethylbenzene have been reported as intermediates in the process. Valentin Koptyug
Valentin Afanasyevich Koptyug (russian: Валентин Афанасьевич Коптюг; June 9, 1931 – January 10, 1997) was a Soviet Belarusian scientist, specializing in physical and organic chemistry.
Biography
Valentin Koptyug was b ...
and co-workers found that both hexamethylcyclohexadienone isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Is ...
s (2,3,4,4,5,6- and 2,3,4,5,6,6-) are intermediates in the process, undergoing methyl migration to form the 1,2,3,4,5,6-hexamethylbenzene carbon skeleton.
Trimerisation of three 2-butyne (dimethylacetylene) molecules yields hexamethylbenzene. The reaction is catalyzed by triphenylchromium tri-tetrahydrofuranate or by a complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
of triisobutylaluminium and titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula . It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. is a volatile liquid. Upon contact with humid air, it forms thick clouds ...
.

Uses
Hexamethylbenzene has no commercial or widespread uses. It is exclusively of interest for chemical research.Reactions
It forms orange-yellow 1:1 adduct withpicryl chloride
Picryl chloride is an organic compound with the formula ClC6H2(NO2)3. It is a bright yellow solid that is highly explosive, as is typical for polynitro aromatics such as picric acid. Its detonation velocity is 7,200 m/s.
Reactions
The reactivit ...
, probably due to π-stacking of the aromatic systems.
Oxidation with trifluoroperacetic acid
Trifluoroperacetic acid (trifluoroperoxyacetic acid, TFPAA) is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula . It is a strong oxidizing agent for organic oxidation reactions, such ...
or hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
gives 2,3,4,5,6,6-hexamethyl-2,4-cyclohexadienone:)
 It has also been used as a solvent for 3He-NMR spectroscopy.
Just as with benzene itself, the electron-rich aromatic system in hexamethylbenzene allows it to act as a
It has also been used as a solvent for 3He-NMR spectroscopy.
Just as with benzene itself, the electron-rich aromatic system in hexamethylbenzene allows it to act as a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
in organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
. The electron-donating nature of the methyl groups—both that there are six of them individually and that there are six ''meta
Meta (from the Greek μετά, '' meta'', meaning "after" or "beyond") is a prefix meaning "more comprehensive" or "transcending".
In modern nomenclature, ''meta''- can also serve as a prefix meaning self-referential, as a field of study or end ...
'' pairs among them—enhance the basicity of the central ring by six to seven orders of magnitude
An order of magnitude is an approximation of the logarithm of a value relative to some contextually understood reference value, usually 10, interpreted as the base of the logarithm and the representative of values of magnitude one. Logarithmic dis ...
relative to benzene. Examples of such complexes have been reported for a variety of metal centres, including cobalt, chromium, iron, rhenium, rhodium, ruthenium, and titanium. Known cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s of sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic derivat ...
es of cobalt and rhodium with hexamethylbenzene take the form (M = Co, Fe, Rh, Ru; ''n'' = 1, 2), where the metal centre is bound by the π electrons of the two arene moieties, and can easily be synthesised from appropriate metal salts by ligand exchange, for example:
: + 2 → + 2
The complexes can undergo redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
reactions. The rhodium and cobalt dications undergo a one-electron reduction with a suitable active metal (aluminium for the cobalt system, zinc for the rhodium), and the equations describing the reactions in the cobalt system are as follows:
:3 + Al → 3 +
 In the field of organoruthenium chemistry, the redox interconversion of the analogous two-electron reduction of the dication and its neutral product occurs at −1.02 V in
In the field of organoruthenium chemistry, the redox interconversion of the analogous two-electron reduction of the dication and its neutral product occurs at −1.02 V in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile ( hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
and is accompanied by a structural change. The hapticity
In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a l ...
of one of the hexamethylbenzene ligands changes with the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of the ruthenium centre, the dication u(η6-C6(CH3)6)2sup>2+ being reduced to u(η4-C6(CH3)6)(η6-C6(CH3)6) with the structural change allowing each complex to comply with the 18-electron rule and maximise stability.
The equivalent iron(II) complex undergoes a reversible one-electron reduction (at −0.48 V in aqueous ethanol), but the two-electron reduction (at −1.46 V) is irreversible, suggesting a change in structure different from that found in the ruthenium system.
Dication
 The isolation of an ion with composition was first reported from investigations of hexamethyl Dewar benzene in the 1960s; a pyramidal structure was suggested based on
The isolation of an ion with composition was first reported from investigations of hexamethyl Dewar benzene in the 1960s; a pyramidal structure was suggested based on NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
evidence and subsequently supported by disordered crystal structure data. In the early 1970s theoretical work led by Hepke Hogeveen predicted the existence of a pyramidal dication , and the suggestion was soon supported by experimental evidence. Spectroscopic
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
investigation of the two-electron oxidation of benzene at very low temperatures (below 4 K) shows that a hexagonal dication forms and then rapidly rearranges into a pyramidal structure:

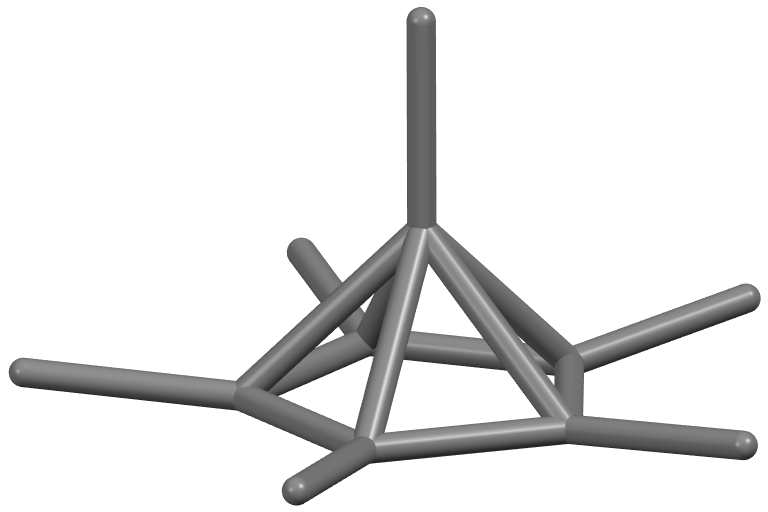
 Two-electron oxidation of hexamethylbenzene would be expected to result in a near-identical rearrangement to a pyramidal carbocation, but attempts to synthesise it in bulk by this method have been unsuccessful. However, a modification of the Hogeveen approach was reported in 2016, along with a high-quality crystal structure determination of . The pyramidal core is about 1.18
Two-electron oxidation of hexamethylbenzene would be expected to result in a near-identical rearrangement to a pyramidal carbocation, but attempts to synthesise it in bulk by this method have been unsuccessful. However, a modification of the Hogeveen approach was reported in 2016, along with a high-quality crystal structure determination of . The pyramidal core is about 1.18 ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
s high, and each of the methyl groups on the ring is located slightly above that base plane to give a somewhat inverted tetrahedral geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are ...
for the carbons of the base of the pyramid. The preparation method involved treating the epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
of hexamethyl Dewar benzene with magic acid
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted– Lewis superacid system was developed in the 1960s ...
, which formally abstracts an oxide anion () to form the dication:
 Though indirect spectroscopic evidence and theoretical calculations previously pointed to their existence, the isolation and structural determination of a species with a hexacoordinate carbon bound only to other carbon atoms is unprecedented, and has attracted comment in ''
Though indirect spectroscopic evidence and theoretical calculations previously pointed to their existence, the isolation and structural determination of a species with a hexacoordinate carbon bound only to other carbon atoms is unprecedented, and has attracted comment in ''Chemical & Engineering News
''Chemical & Engineering News'' (''C&EN'') is a weekly news magazine published by the American Chemical Society, providing professional and technical news and analysis in the fields of chemistry and chemical engineering.New Scientist
''New Scientist'' is a magazine covering all aspects of science and technology. Based in London, it publishes weekly English-language editions in the United Kingdom, the United States and Australia. An editorially separate organisation publish ...
'', ''Science News
''Science News (SN)'' is an American bi-weekly magazine devoted to articles about new scientific and technical developments, typically gleaned from recent scientific and technical journals.
History
''Science News'' has been published since ...
'', and ZME Science. The carbon atom at the top of the pyramid is bonding with six other atoms, an unusual arrangement as the usual maximum valence for this element is four. The molecule is aromatic and avoids exceeding the octet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 com ...
on carbon by having only a total of six electrons in the five bonds between the base of the pyramid and its apex. That is, each of the vertical edges of the pyramid is only a partial bond rather than a normal covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
that would have two electrons shared between two atoms. Although the top carbon does bond to six others, it does so using a total of no more than eight electrons.
The dication, noting the weak bonds forming the upright edges of the pyramid, shown as dashed lines in the structure, have a Wiberg bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs ( covalent bonds) between two atoms. For example, in diat ...
of about 0.54; it follows that the total bond order is 5 × 0.54 + 1 = 3.7 < 4, and thus the species is not hypervalent
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pe ...
, though it is hypercoordinate. The differences in bonding in the dication—the ring having aromatic character and the vertical edges being weak partial bonds—are reflected in variations of the carbon–carbon bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
s: the ring bonds are , the bonds to the methyl groups are , and the vertical edges are Bachrach rationalised the three-dimensional aromaticity of the dication by considering it as comprising the ring as a four-electron donor and topped by the fragment, which provides two electrons, for a total of six electrons in the aromatic cage, in line with Hückel's rule for ''n'' = 1. From the perspective of organometallic chemistry, the species can be viewed as This satisfies the octet rule by binding a carbon(IV) centre () to an aromatic η5– pentamethylcyclopentadienyl anion (six-electron donor) and methyl anion (two-electron donor), analogous to the way the gas-phase organozinc
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compou ...
monomer having the same ligands bound to a zinc(II) centre () satisfies the 18 electron rule on the metal.
It has been commented that " 's super important that people realize that, although we're taught carbon can only have four friends, carbon can be associated with more than four atoms" and added that the "carbon isn't making six bonds in the sense that we usually think of a carbon-carbon bond as a two-electron bond." "It is all about the challenge and the possibility to astonish chemists about what can be possible."
References
{{Hydrocarbons Alkylbenzenes Ligands