hemiaminal on:
[Wikipedia]
[Google]
[Amazon]
In
 The
The 3 RCHO + 3 NH3 -> (RCHNH)3 + 3 H2O
'' -trisubstituted hexahydro-1,3,5-triazines arise from the condensation of the 3 CH2O + 3 H2NMe -> (CH2NMe)3 + 3 H2O
Although adducts generated from primary amines or ammonia are usually unstable, the hemiaminals have been trapped in a cavity.
Me2NH + CH2O -> Me2NCH2OH
:Me2NH + Me2NCH2OH -> Me2NCH2NMe2 + H2O
The reaction of  Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Methanolamine.svg, methanolamine, an intermediate in the reaction of ammonia with formaldehyde
OC(NHCH2OH)2.png, Bis(hydroxymethyl)urea is a commercially useful hemiaminal
CF3-stabilizedHemiaminal.svg, An unusual example of an isolable, acyclic hemiaminal: the adduct of ammonia and
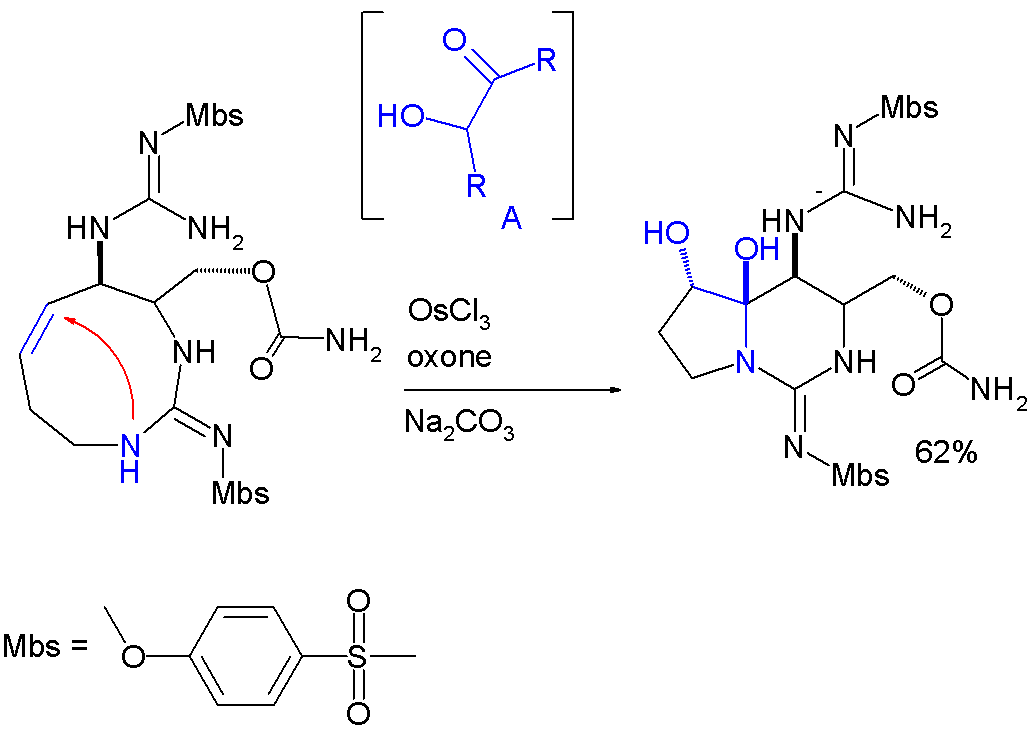 In this reaction step the
In this reaction step the
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, a hemiaminal (also carbinolamine) is a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
or type of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
that has a hydroxyl group and an amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
attached to the same carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atom: . R can be hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
or an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
group. Hemiaminals are intermediates in imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
formation from an amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
and a carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
by alkylimino-de-oxo-bisubstitution
In organic chemistry, alkylimino-de-oxo-bisubstitution is the organic reaction of carbonyl compounds with amines to imines. The reaction name is based on the IUPAC Nomenclature for Transformations. The reaction is acid catalyzed and the reaction ...
. Hemiaminals can be viewed as a blend of aminal
In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: . (As is customary in organic chemistry, R can represent hydrogen or an alkyl group). A ...
s and geminal diol
In chemistry, the descriptor geminal () refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol (a molecule that has two alcohol functional groups) attached t ...
. They are a special case of amino alcohol
In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.
Meth ...
s.
Classification according to amine precursor
Addition of ammonia
 The
The adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all co ...
s formed by the addition of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
to aldehydes have long been studied. Compounds containing both a primary amino
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
group and a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
group bonded to the same carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atom are rare. They are invoked but rarely observed as intermediates in the reaction of ammonia and aldehydes and ketones. One example of this rare functionality is the adduct of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
and hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a mus ...
, .
The C-substituted derivatives are obtained by reaction of aldehydes and ammonia:
:Addition of primary amines
N-substituted derivatives are somewhat stable. These N,N',Namine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
and formaldehyde as illustrated by the route to 1,3,5-trimethyl-1,3,5-triazacyclohexane:
:Addition of secondary amines: carbinolamines (hemiaminals) and bisaminomethanes
One of the simplest reactions entails condensation of formaldehyde and dimethylamine. This reaction produces first the carbinolamine (a hemiaminal) and bis(dimethylamino)methane (): :formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
with carbazole
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole str ...
, which is weakly basic, proceed similarly:
: Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Again, this carbinol converts readily to the methylene-linked bis(carbazole).
Hemiaminal ethers
Hemiaminal ethers have the following structure: R‴-C(NR'2)(OR")-R⁗. The glycosylamines are examples of cyclic hemiaminal ethers.hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a mus ...
Hemiaminal ether aldehyde.png, Hemiaminal ether derived from an aldehyde
Hemiaminal ether ketone.png, Hemiaminal ether derived from a ketone
T-BuOCH(NMe2)2.svg, Tert-Butoxybis(dimethylamino)methane
Use in total synthesis
Hemiaminal formation is a key step in an asymmetrictotal synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes i ...
of saxitoxin:
: In this reaction step the
In this reaction step the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
group is first oxidized to an intermediate acyloin
Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl grou ...
by action of osmium(III) chloride, oxone ( sacrificial catalyst) and sodium carbonate (base).
See also
*Aminal
In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: . (As is customary in organic chemistry, R can represent hydrogen or an alkyl group). A ...
*Alkanolamine
In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.
...
*Hemiacetal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemi ...
References
{{Commonscat, Hemiaminals Functional groups