Glacial Acetic Acid on:
[Wikipedia]
[Google]
[Amazon]
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and
Chapter P-12.1; page 4
/ref> The name "acetic acid" derives from "acetum", the



 Acetic acid is produced industrially both synthetically and by bacterial
Acetic acid is produced industrially both synthetically and by bacterial
 The process involves iodomethane as an intermediate, and occurs in three steps. A
The process involves iodomethane as an intermediate, and occurs in three steps. A
 In 1845 German chemist Hermann Kolbe synthesised acetic acid from
In 1845 German chemist Hermann Kolbe synthesised acetic acid from
National Pollutant Inventory – Acetic acid fact sheetMethod for sampling and analysis29 CFR 1910.1000, Table Z-1
(US Permissible exposure limits)
*Calculation o
vapor pressureliquid densitydynamic liquid viscositysurface tension
of acetic acid
Acetic acid bound to proteins
in the PDB
Swedish Chemicals Agency. Information sheet – Acetic Acid
* Process Flow sheet of Acetic acid Production by th
Carbonylation of Methanol
{{portal bar, Medicine Acids in wine Antiseptics Commodity chemicals E-number additives Flavors Household chemicals Otologicals Photographic chemicals Solvents World Health Organization essential medicines Organic compounds with 2 carbon atoms
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
(also written as , , or ). Vinegar
Vinegar is an aqueous solution of acetic acid and trace compounds that may include flavorings. Vinegar typically contains 5–8% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting simple sugars to eth ...
is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.
Acetic acid is the second simplest carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
(after formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Est ...
). It is an important chemical reagent and industrial chemical, used primarily in the production of cellulose acetate
In biochemistry, cellulose acetate refers to any acetate ester of cellulose, usually cellulose diacetate. It was first prepared in 1865. A bioplastic, cellulose acetate is used as a film base in photography, as a component in some coatings, and ...
for photographic film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the crystals determine ...
, polyvinyl acetate
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue, PVA glue, white glue, carpenter's glue, school glue, or Elmer's glue in the US, is a widely available adhesive used for porous materials like wood, paper, and ...
for wood glue
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advant ...
, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry
The food industry is a complex, global network of diverse businesses that supplies most of the food consumed by the world's population. The food industry today has become highly diversified, with manufacturing ranging from small, traditional, ...
, acetic acid is controlled by the food additive code E260 as an acidity regulator
Acidity regulators, or pH control agents, are food additives used to change or maintain pH (acidity or basicity). They can be organic or mineral acids, bases, neutralizing agents, or buffering agents. Typical agents include the following acids ...
and as a condiment. In biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, the acetyl group
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, ...
, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a subs ...
, it is central to the metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run ...
of carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may o ...
s and fats.
The global demand for acetic acid is about 6.5 million metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
per year (t/a), of which approximately 1.5 t/a is met by recycling; the remainder is manufactured from methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
. Vinegar is mostly dilute acetic acid, often produced by fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food p ...
and subsequent oxidation of ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
.
Nomenclature
Thetrivial name
In chemistry, a trivial name is a non systematic name for a chemical substance. That is, the name is not recognized according to the rules of any formal system of chemical nomenclature such as IUPAC inorganic or IUPAC organic nomenclature. A ...
"acetic acid" is the most commonly used and preferred IUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
. The systematic name "ethanoic acid", a valid IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
name, is constructed according to the substitutive nomenclature.IUPAC Provisional Recommendations 200Chapter P-12.1; page 4
/ref> The name "acetic acid" derives from "acetum", the
Latin
Latin (, or , ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through ...
word for vinegar
Vinegar is an aqueous solution of acetic acid and trace compounds that may include flavorings. Vinegar typically contains 5–8% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting simple sugars to eth ...
, and is related to the word "acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
" itself.
"Glacial acetic acid" is a name for water-free (anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achi ...
) acetic acid. Similar to the German
German(s) may refer to:
* Germany (of or related to)
**Germania (historical use)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizens of Germany, see also German nationality law
**Ge ...
name "Eisessig" ("ice vinegar"), the name comes from the ice-like crystals that form slightly below room temperature at (the presence of 0.1% water lowers its melting point by 0.2 °C).
A common symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
for acetic acid is AcOH, where Ac is the pseudoelement symbol representing the acetyl
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, ...
group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic ide ...
; the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
, acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
(), is thus represented as . (The symbol Ac for the acetyl functional group is not to be confused with the symbol Ac for the element actinium
Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance ...
; the context prevents confusion among organic chemists). To better reflect its structure, acetic acid is often written as , , , and . In the context of acid–base reaction
An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their appl ...
s, the abbreviation HAc is sometimes used, where Ac in this case is a symbol for acetate (rather than acetyl). Acetate is the ion resulting from loss of from acetic acid. The name "acetate" can also refer to a salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
containing this anion, or an ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
of acetic acid.
Properties

Acidity
The hydrogen centre in thecarboxyl group
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
(−COOH) in carboxylic acids such as acetic acid can separate from the molecule by ionization:
:
Because of this release of the proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
(), acetic acid has acidic character. Acetic acid is a weak monoprotic acid. In aqueous solution, it has a pKa value of 4.76. Its conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
is acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
(). A 1.0 M solution (about the concentration of domestic vinegar) has a pH of 2.4, indicating that merely 0.4% of the acetic acid molecules are dissociated. However, in very dilute (< 10−6 M) solution acetic acid is >90% dissociated.

Structure
In solid acetic acid, the molecules form chains, individual molecules being interconnected byhydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
s. In the vapour at , dimers can be detected. Dimers also occur in the liquid phase in dilute solutions in non-hydrogen-bonding solvents, and a certain extent in pure acetic acid, but are disrupted by hydrogen-bonding solvents. The dissociation enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
of the dimer is estimated at 65.0–66.0 kJ/mol, and the dissociation entropy at 154–157 J mol−1 K−1. Other carboxylic acids engage in similar intermolecular hydrogen bonding interactions.
Solvent properties
Liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, ...
acetic acid is a hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
(polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
*Polar climate, the cli ...
) protic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile ...
, similar to ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. With a relative static permittivity
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insul ...
(dielectric constant) of 6.2, it dissolves not only polar compounds such as inorganic salts and sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or do ...
s, but also non-polar compounds such as oils as well as polar solutes. It is miscible with polar and non-polar solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s such as water, chloroform
Chloroform, or trichloromethane, is an organic compound with formula C H Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various ...
, and hexane
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relative ...
. With higher alkanes (starting with octane
Octane is a hydrocarbon and an alkane with the chemical formula , and the condensed structural formula . Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. One of these isomers, 2,2,4-t ...
), acetic acid is not miscible
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also applies ...
at all compositions, and solubility of acetic acid in alkanes declines with longer n-alkanes. The solvent and miscibility
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). The term is most often applied to liquids but also appl ...
properties of acetic acid make it a useful industrial chemical, for example, as a solvent in the production of dimethyl terephthalate
Dimethyl terephthalate (DMT) is an organic compound with the formula C6H4(COOCH3)2. It is the diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.Richard J. Sheehan "Terepht ...
.
Biochemistry
At physiological pHs, acetic acid is usually fully ionised toacetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
.
The acetyl
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, ...
group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic ide ...
, formally derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a subs ...
, it is central to the metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run ...
of carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may o ...
s and fats. Unlike longer-chain carboxylic acids (the fatty acids
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an B ...
), acetic acid does not occur in natural triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''tri-'' and ''glyceride'').
Triglycerides are the main constituents of body fat in humans and other vertebrates, as ...
s. However, the artificial triglyceride triacetin
Triacetin, is the organic compound with the formula . It is classified as a triglyceride, i.e., the triester of glycerol. It is a colorless, viscous, and odorless liquid with a high boiling point and a low melting point. It has a mild, sweet tast ...
(glycerine triacetate) is a common food additive and is found in cosmetics and topical medicines.
Acetic acid is produced and excreted by acetic acid bacteria
Acetic acid bacteria (AAB) are a group of Gram-negative bacteria which oxidize sugars or ethanol and produce acetic acid during fermentation. The acetic acid bacteria consist of 10 genera in the family Acetobacteraceae. Several species of acetic ...
, notably the genus '' Acetobacter'' and ''Clostridium acetobutylicum
''Clostridium acetobutylicum'', ATCC 824, is a commercially valuable bacterium sometimes called the "Weizmann Organism", after Jewish Russian-born biochemist Chaim Weizmann. A senior lecturer at the University of Manchester, England, he used th ...
''. These bacteria are found universally in food
Food is any substance consumed by an organism for nutritional support. Food is usually of plant, animal, or fungal origin, and contains essential nutrients, such as carbohydrates, fats, proteins, vitamins, or minerals. The substance is in ...
stuffs, water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
, and soil
Soil, also commonly referred to as earth or dirt, is a mixture of organic matter, minerals, gases, liquids, and organisms that together support life. Some scientific definitions distinguish ''dirt'' from ''soil'' by restricting the former ...
, and acetic acid is produced naturally as fruits and other foods spoil. Acetic acid is also a component of the vaginal lubrication
Vaginal lubrication is a naturally produced fluid that lubricates a
vagina. Vaginal lubrication is always present, but production increases significantly near ovulation and during sexual arousal in anticipation of sexual intercourse. Vaginal ...
of human
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, cultu ...
s and other primate
Primates are a diverse order of mammals. They are divided into the strepsirrhines, which include the lemurs, galagos, and lorisids, and the haplorhines, which include the tarsiers and the simians ( monkeys and apes, the latter includin ...
s, where it appears to serve as a mild antibacterial
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention ...
agent.
Production
 Acetic acid is produced industrially both synthetically and by bacterial
Acetic acid is produced industrially both synthetically and by bacterial fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food p ...
. About 75% of acetic acid made for use in the chemical industry is made by the carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbon ...
of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
, explained below. The biological route accounts for only about 10% of world production, but it remains important for the production of vinegar because many food purity laws require vinegar used in foods to be of biological origin. Other processes are methyl formate isomerization, conversion of syngas to acetic acid, and gas phase oxidation of ethylene and ethanol. Acetic acid is often a side product of different reactions, e.g. during heterogeneous catalytic acrylic acid synthesis or fermentative lactic acid production. Acetic acid can be purified via fractional freezing
Fractional freezing is a process used in process engineering and chemistry to separate substances with different melting points. It can be done by partial melting of a solid, for example in zone refining of silicon or metals, or by partial crys ...
using an ice bath. The water and other impurities
In chemistry and materials science, impurities are chemical substances inside a confined amount of liquid, gas, or solid, which differ from the chemical composition of the material or compound. Firstly, a pure chemical should appear thermodynami ...
will remain liquid while the acetic acid will precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading ...
out. As of 2003–2005, total worldwide production of virgin acetic acid was estimated at 5 Mt/a (million tonnes per year), approximately half of which was produced in the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country Continental United States, primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., ...
. Europe
Europe is a large peninsula conventionally considered a continent in its own right because of its great physical size and the weight of its history and traditions. Europe is also considered a Continent#Subcontinents, subcontinent of Eurasia ...
an production was approximately 1 Mt/a and declining, while Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the n ...
ese production was 0.7 Mt/a. Another 1.5 Mt were recycled each year, bringing the total world market to 6.5 Mt/a. Since then the global production has increased to 10.7 Mt/a (in 2010), and further; however, a slowing in this increase in production is predicted. The two biggest producers of virgin acetic acid are Celanese
Celanese Corporation, formerly known as Hoechst Celanese, is an American technology and specialty materials company headquartered in Irving, Texas. A Fortune 500 corporation, the company is the world’s leading producer of acetic acid, produ ...
and BP Chemicals. Other major producers include Millennium Chemicals
Millennium Inorganic Chemicals is a Hunt Valley, Maryland based chemical company.
The business was established in 1985. It was a subsidiary of British conglomerate Hanson plc at one time, but was demerged on 1 October 1996, when it became an i ...
, Sterling Chemicals, Samsung
The Samsung Group (or simply Samsung) ( ko, 삼성 ) is a South Korean multinational manufacturing conglomerate headquartered in Samsung Town, Seoul, South Korea. It comprises numerous affiliated businesses, most of them united under the ...
, Eastman, and Svensk Etanolkemi
Swedish or ' may refer to:
Anything from or related to Sweden, a country in Northern Europe. Or, specifically:
* Swedish language, a North Germanic language spoken primarily in Sweden and Finland
** Swedish alphabet, the official alphabet used by ...
.
Methanol carbonylation
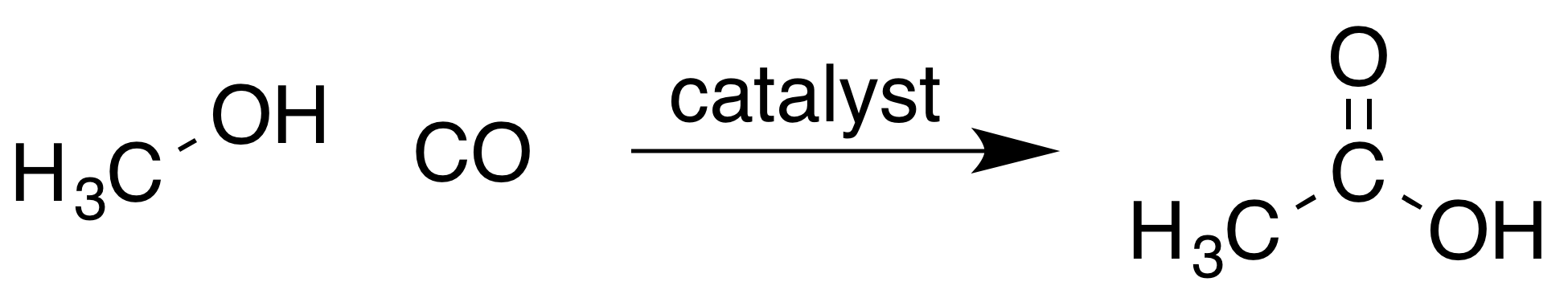
Most acetic acid is produced by methanolcarbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbon ...
. In this process, methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
and carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
react to produce acetic acid according to the equation:
: The process involves iodomethane as an intermediate, and occurs in three steps. A
The process involves iodomethane as an intermediate, and occurs in three steps. A catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe c ...
, is needed for the carbonylation (step 2).
#
#
#
Two related processes exist for the carbonylation of methanol: the rhodium-catalyzed Monsanto process, and the iridium-catalyzed Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an ...
. The latter process is greener and more efficient and has largely supplanted the former process, often in the same production plants. Catalytic amounts of water are used in both processes, but the Cativa process requires less, so the water-gas shift reaction is suppressed, and fewer by-products are formed.
By altering the process conditions, acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a co ...
may also be produced on the same plant using the rhodium catalysts.
Acetaldehyde oxidation
Prior to the commercialization of the Monsanto process, most acetic acid was produced by oxidation ofacetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
. This remains the second-most-important manufacturing method, although it is usually not competitive with the carbonylation of methanol. The acetaldehyde can be produced by hydration of acetylene. This was the dominant technology in the early 1900s.
Light naphtha
Naphtha ( or ) is a flammable liquid hydrocarbon mixture.
Mixtures labelled ''naphtha'' have been produced from natural gas condensates, petroleum distillates, and the distillation of coal tar and peat. In different industries and regions ' ...
components are readily oxidized by oxygen or even air to give peroxides
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
, which decompose to produce acetic acid according to the chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
, illustrated with butane:
:
Such oxidations require metal catalyst, such as the naphthenate salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
s of manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
, and chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
.
The typical reaction is conducted at temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
s and pressures designed to be as hot as possible while still keeping the butane a liquid. Typical reaction conditions are and 55 atm. Side-products may also form, including butanone
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in ...
, ethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Est ...
, and propionic acid
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a li ...
. These side-products are also commercially valuable, and the reaction conditions may be altered to produce more of them where needed. However, the separation of acetic acid from these by-products adds to the cost of the process.
Under similar conditions and using similar catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s as are used for butane oxidation, the oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
in air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
to produce acetic acid can oxidize acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
.
:
Using modern catalysts, this reaction can have an acetic acid yield greater than 95%. The major side-products are ethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Est ...
, and formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
, all of which have lower boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding env ...
s than acetic acid and are readily separated by distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
.
Ethylene oxidation
Acetaldehyde may be prepared fromethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
via the Wacker process, and then oxidised as above.
In more recent times, chemical company Showa Denko, which opened an ethylene oxidation plant in Ōita, Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the n ...
, in 1997, commercialised a cheaper single-stage conversion of ethylene to acetic acid. The process is catalyzed by a palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
metal catalyst supported on a heteropoly acid such as silicotungstic acid
Silicotungstic acid or tungstosilicic acid is a heteropoly acid with the chemical formula . It forms hydrates . In freshly prepared samples, ''n'' is approximately 29, but after prolonged desiccation, ''n'' = 6. It is a white solid although impu ...
. A similar process uses the same metal catalyst on silicotungstic acid and silica:
:
It is thought to be competitive with methanol carbonylation for smaller plants (100–250 kt/a), depending on the local price of ethylene.
The approach will be based on utilizing a novel selective photocatalytic oxidation technology for the selective oxidation of ethylene and ethane to acetic acid. Unlike traditional oxidation catalysts, the selective oxidation process will use UV light to produce acetic acid at ambient temperatures and pressure.
Oxidative fermentation
For most of human history, acetic acid bacteria of the genus '' Acetobacter'' have made acetic acid, in the form of vinegar. Given sufficient oxygen, these bacteria can produce vinegar from a variety of alcoholic foodstuffs. Commonly used feeds includeapple cider
Apple cider (also called sweet cider, soft cider, or simply cider) is the name used in the United States and Canada for an unfiltered, unsweetened, non-alcoholic beverage made from apples. Though typically referred to simply as "cider" in the U ...
, wine
Wine is an alcoholic drink typically made from Fermentation in winemaking, fermented grapes. Yeast in winemaking, Yeast consumes the sugar in the grapes and converts it to ethanol and carbon dioxide, releasing heat in the process. Different ...
, and fermented grain
A grain is a small, hard, dry fruit ( caryopsis) – with or without an attached hull layer – harvested for human or animal consumption. A grain crop is a grain-producing plant. The two main types of commercial grain crops are cereals and legum ...
, malt
Malt is germinated cereal grain that has been dried in a process known as " malting". The grain is made to germinate by soaking in water and is then halted from germinating further by drying with hot air.
Malted grain is used to make beer, w ...
, rice
Rice is the seed of the grass species '' Oryza sativa'' (Asian rice) or less commonly ''Oryza glaberrima'' (African rice). The name wild rice is usually used for species of the genera '' Zizania'' and '' Porteresia'', both wild and domesticat ...
, or potato
The potato is a starchy food, a tuber of the plant ''Solanum tuberosum'' and is a root vegetable native to the Americas. The plant is a perennial in the nightshade family Solanaceae.
Wild potato species can be found from the southern Uni ...
mashes. The overall chemical reaction facilitated by these bacteria is:
:
A dilute alcohol solution inoculated with ''Acetobacter'' and kept in a warm, airy place will become vinegar over the course of a few months. Industrial vinegar-making methods accelerate this process by improving the supply of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
to the bacteria.
The first batches of vinegar produced by fermentation probably followed errors in the winemaking
Winemaking or vinification is the production of wine, starting with the selection of the fruit, its fermentation into alcohol, and the bottling of the finished liquid. The history of wine-making stretches over millennia. The science of wine and ...
process. If must
Must (from the Latin ''vinum mustum'', "young wine") is freshly crushed fruit juice (usually grape juice) that contains the skins, seeds, and stems of the fruit. The solid portion of the must is called pomace and typically makes up 7–23% of th ...
is fermented at too high a temperature, acetobacter will overwhelm the yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constit ...
naturally occurring on the grapes
A grape is a fruit, botanically a berry, of the deciduous woody vines of the flowering plant genus ''Vitis''. Grapes are a non- climacteric type of fruit, generally occurring in clusters.
The cultivation of grapes began perhaps 8,000 years ago, ...
. As the demand for vinegar for culinary, medical, and sanitary purposes increased, vintners quickly learned to use other organic materials to produce vinegar in the hot summer months before the grapes were ripe and ready for processing into wine. This method was slow, however, and not always successful, as the vintners did not understand the process.
One of the first modern commercial processes was the "fast method" or "German method", first practised in Germany in 1823. In this process, fermentation takes place in a tower packed with wood shavings or charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, ...
. The alcohol-containing feed is trickled into the top of the tower, and fresh air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
supplied from the bottom by either natural or forced convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the c ...
. The improved air supply in this process cut the time to prepare vinegar from months to weeks.
Nowadays, most vinegar is made in submerged tank culture
Culture () is an umbrella term which encompasses the social behavior, institutions, and norms found in human societies, as well as the knowledge, beliefs, arts, laws, customs, capabilities, and habits of the individuals in these groups ...
, first described in 1949 by Otto Hromatka and Heinrich Ebner. In this method, alcohol is fermented to vinegar in a continuously stirred tank, and oxygen is supplied by bubbling air through the solution. Using modern applications of this method, vinegar of 15% acetic acid can be prepared in only 24 hours in batch process, even 20% in 60-hour fed-batch process.
Anaerobic fermentation
Species ofanaerobic bacteria
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenat ...
, including members of the genus ''Clostridium
''Clostridium'' is a genus of anaerobic, Gram-positive bacteria. Species of ''Clostridium'' inhabit soils and the intestinal tract of animals, including humans. This genus includes several significant human pathogens, including the causative a ...
'' or '' Acetobacterium'' can convert sugars to acetic acid directly without creating ethanol as an intermediate. The overall chemical reaction conducted by these bacteria may be represented as:
:
These acetogen
An acetogen is a microorganism that generates acetate (CH3COO−) as an end product of anaerobic respiration or fermentation. However, this term is usually employed in a narrower sense only to those bacteria and archaea that perform anaerobic resp ...
ic bacteria produce acetic acid from one-carbon compounds, including methanol, carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
, or a mixture of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
:
:
This ability of ''Clostridium'' to metabolize sugars directly, or to produce acetic acid from less costly inputs, suggests that these bacteria could produce acetic acid more efficiently than ethanol-oxidizers like ''Acetobacter''. However, ''Clostridium'' bacteria are less acid-tolerant than ''Acetobacter''. Even the most acid-tolerant ''Clostridium'' strains can produce vinegar in concentrations of only a few per cent, compared to ''Acetobacter'' strains that can produce vinegar in concentrations up to 20%. At present, it remains more cost-effective to produce vinegar using ''Acetobacter'', rather than using ''Clostridium'' and concentrating it. As a result, although acetogenic bacteria have been known since 1940, their industrial use is confined to a few niche applications.
Uses
Acetic acid is a chemicalreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
for the production of chemical compounds. The largest single use of acetic acid is in the production of vinyl acetate monomer
In chemistry, a monomer ( ; '' mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
, closely followed by acetic anhydride and ester production. The volume of acetic acid used in vinegar is comparatively small.
Vinyl acetate monomer
The primary use of acetic acid is the production ofvinyl acetate
Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate and ethene-vinyl acetate copolymers, important industrial polymers.
Production
The worldwide production capacity of v ...
monomer (VAM). In 2008, this application was estimated to consume a third of the world's production of acetic acid. The reaction consists of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
and acetic acid with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
over a palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, conducted in the gas phase.
:
Vinyl acetate can be polymerised to polyvinyl acetate
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue, PVA glue, white glue, carpenter's glue, school glue, or Elmer's glue in the US, is a widely available adhesive used for porous materials like wood, paper, and ...
or other polymers
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
, which are components in paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s and adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advant ...
s.
Ester production
The majorester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s of acetic acid are commonly used as solvents for inks, paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s and coating
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. Coatings may be applied as liquids, gases or solids e.g. Pow ...
s. The esters include ethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
, ''n''-butyl acetate
''n''-Butyl acetate is an organic compound with the formula . A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smel ...
, isobutyl acetate
The chemical compound isobutyl acetate, also known as 2-methylpropyl ethanoate (IUPAC name) or β-methylpropyl acetate, is a common solvent. It is produced from the esterification of isobutanol with acetic acid. It is used as a solvent for lacque ...
, and propyl acetate
Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used i ...
. They are typically produced by catalyzed reaction from acetic acid and the corresponding alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
:
:, R = general alkyl group
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
For example, acetic acid and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
gives ethyl acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
.
:
Most acetate esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
, however, are produced from acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
using the Tishchenko reaction The Tishchenko reaction is an organic chemical reaction that involves disproportionation of an aldehyde in the presence of an alkoxide. The reaction is named after Russian organic chemist Vyacheslav Tishchenko, who discovered that aluminium alkoxi ...
. In addition, ether acetates are used as solvents for nitrocellulose
Nitrocellulose (also known as cellulose nitrate, flash paper, flash cotton, guncotton, pyroxylin and flash string, depending on form) is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and ...
, acrylic lacquer
Lacquer is a type of hard and usually shiny coating or finish applied to materials such as wood or metal. It is most often made from resin extracted from trees and waxes and has been in use since antiquity.
Asian lacquerware, which may be ca ...
s, varnish
Varnish is a clear transparent hard protective coating or film. It is not a stain. It usually has a yellowish shade from the manufacturing process and materials used, but it may also be pigmented as desired, and is sold commercially in variou ...
removers, and wood stains. First, glycol monoethers are produced from ethylene oxide
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sw ...
or propylene oxide
Propylene oxide is an acutely toxic and carcinogenic organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid with an odour similar to ether, is produced on a large scale industrially. Its major application is its us ...
with alcohol, which are then esterified with acetic acid. The three major products are ethylene glycol monoethyl ether acetate (EEA), ethylene glycol monobutyl ether acetate (EBA), and propylene glycol monomethyl ether acetate (PMA, more commonly known as PGMEA in semiconductor manufacturing processes, where it is used as a resist solvent). This application consumes about 15% to 20% of worldwide acetic acid. Ether acetates, for example EEA, have been shown to be harmful to human reproduction.
Acetic anhydride
The product of thecondensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapo ...
of two molecules of acetic acid is acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a co ...
. The worldwide production of acetic anhydride is a major application, and uses approximately 25% to 30% of the global production of acetic acid. The main process involves dehydration of acetic acid to give ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound ethen ...
at 700–750 °C. Ketene is thereafter reacted with acetic acid to obtain the anhydride:
:
:
Acetic anhydride is an acetylation
:
In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply '' acetates''. Deacetylation is the oppos ...
agent. As such, its major application is for cellulose acetate
In biochemistry, cellulose acetate refers to any acetate ester of cellulose, usually cellulose diacetate. It was first prepared in 1865. A bioplastic, cellulose acetate is used as a film base in photography, as a component in some coatings, and ...
, a synthetic textile
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, different #Fabric, fabric types, etc. At f ...
also used for photographic film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the crystals determine ...
. Acetic anhydride is also a reagent for the production of heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a potent opioid mainly used as a recreational drug for its euphoric effects. Medical grade diamorphine is used as a pure hydrochloride salt. Various white and bro ...
and other compounds.
Use as solvent
As a polarprotic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile ...
, acetic acid is frequently used for recrystallization to purify organic compounds. Acetic acid is used as a solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
in the production of terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced an ...
(TPA), the raw material for polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and food ...
(PET). In 2006, about 20% of acetic acid was used for TPA production.
Acetic acid is often used as a solvent for reactions involving carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encount ...
s, such as Friedel-Crafts alkylation. For example, one stage in the commercial manufacture of synthetic camphor
Camphor () is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel (''Cinnamomum camphora''), a large evergreen tree found in East Asia; and in the k ...
involves a Wagner-Meerwein rearrangement of camphene to isobornyl acetate; here acetic acid acts both as a solvent and as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
to trap the rearranged carbocation.
Glacial acetic acid is used in analytical chemistry for the estimation of weakly alkaline substances such as organic amides. Glacial acetic acid is a much weaker base than water, so the amide behaves as a strong base in this medium. It then can be titrated using a solution in glacial acetic acid of a very strong acid, such as perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueo ...
.
Medical use
Acetic acid injection into a tumor has been used to treat cancer since the 1800s. Acetic acid is used as part ofcervical cancer screening
Cervical screening is the process of detecting and removing abnormal tissue or cells in the cervix before cervical cancer develops. By aiming to detect and treat cervical neoplasia early on, cervical screening aims at secondary prevention of cervi ...
in many areas in the developing world
A developing country is a sovereign state with a lesser developed industrial base and a lower Human Development Index (HDI) relative to other countries. However, this definition is not universally agreed upon. There is also no clear agreeme ...
. The acid is applied to the cervix
The cervix or cervix uteri (Latin, 'neck of the uterus') is the lower part of the uterus (womb) in the human female reproductive system. The cervix is usually 2 to 3 cm long (~1 inch) and roughly cylindrical in shape, which changes during ...
and if an area of white appears after about a minute the test is positive.
Acetic acid is an effective antiseptic when used as a 1% solution, with broad spectrum of activity against streptococci, staphylococci, pseudomonas, enterococci and others. It may be used to treat skin infections caused by pseudomonas strains resistant to typical antibiotics.
While diluted acetic acid is used in iontophoresis
Iontophoresis is a process of transdermal drug delivery by use of a voltage gradient on the skin. Molecules are transported across the stratum corneum by electrophoresis and electroosmosis and the electric field can also increase the permeability ...
, no high quality evidence supports this treatment for rotator cuff disease.
As a treatment for otitis externa
Otitis externa, also called swimmer's ear, is inflammation of the ear canal. It often presents with ear pain, swelling of the ear canal, and occasionally decreased hearing. Typically there is pain with movement of the outer ear. A high fever is ...
, it is on the World Health Organization's List of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health s ...
.
Foods
Acetic acid has per 100 g. Vinegar is typically no less than 4% acetic acid by mass. Legal limits on acetic acid content vary by jurisdiction. Vinegar is used directly as acondiment
A condiment is a preparation that is added to food, typically after cooking, to impart a specific flavor, to enhance the flavor, or to complement the dish. A table condiment or table sauce is more specifically a condiment that is served separat ...
, and in the pickling
Pickling is the process of preserving or extending the shelf life of food by either anaerobic fermentation in brine or immersion in vinegar. The pickling procedure typically affects the food's texture and flavor. The resulting food is cal ...
of vegetables and other foods. Table vinegar tends to be more diluted (4% to 8% acetic acid), while commercial food pickling employs solutions that are more concentrated. The proportion of acetic acid used worldwide as vinegar is not as large as commercial uses, but is by far the oldest and best-known application.
Reactions
Organic chemistry
Acetic acid undergoes the typicalchemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
s of a carboxylic acid. Upon treatment with a standard base, it converts to metal acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
and water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
. With strong bases (e.g., organolithium reagents), it can be doubly deprotonated to give . Reduction of acetic acid gives ethanol. The OH group is the main site of reaction, as illustrated by the conversion of acetic acid to acetyl chloride
Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
Synthesis
On a ...
. Other substitution derivatives include acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a co ...
; this anhydride
An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the pa ...
is produced by loss of water from two molecules of acetic acid. Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s of acetic acid can likewise be formed via Fischer esterification
Fischer is a German occupational surname, meaning fisherman. The name Fischer is the fourth most common German surname. The English version is Fisher.
People with the surname A
* Abraham Fischer (1850–1913) South African public official
* ...
, and amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
s can be formed. When heated above , acetic acid decomposes to produce carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
and methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane ...
, or to produce ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound ethen ...
and water:
:
:
Reactions with inorganic compounds
Acetic acid is mildlycorrosive
A corrosive substance is one that will damage or destroy other substances with which it comes into contact by means of a chemical reaction.
Etymology
The word ''corrosive'' is derived from the Latin verb ''corrodere'', which means ''to gnaw'', ...
to metal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
s including iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, forming hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
gas and salts called acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
s:
:
Because aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
forms a passivating acid-resistant film of aluminium oxide, aluminium tanks are used to transport acetic acid. Metal acetates can also be prepared from acetic acid and an appropriate base, as in the popular "baking soda
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3� ...
+ vinegar" reaction giving off sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
:
:
A colour reaction In chemistry, a color reaction or colour reaction is a chemical reaction that is used to transform colorless chemical compounds into colored derivatives which can be detected visually or with the aid of a colorimeter.
The concentration of a color ...
for salts of acetic acid is iron(III) chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The col ...
solution, which results in a deeply red colour that disappears after acidification. A more sensitive test uses lanthanum nitrate with iodine and ammonia to give a blue solution. Acetates when heated with arsenic trioxide
Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound and medication. As an industrial chemical, whose major uses include in the manufacture of wood preservatives, pesticides, and glass. As a medication, it ...
form cacodyl oxide
Cacodyl oxide is a chemical compound of the formula CH3)2Assub>2O. This organoarsenic compound is primarily of historical significance since it is sometimes considered to be the first organometallic compound synthesized in relatively pure form. ...
, which can be detected by its malodorous vapours.
Other derivatives
Organic or inorganic salts are produced from acetic acid. Some commercially significant derivatives: *Sodium acetate
Sodium acetate, CH3COONa, also abbreviated Na O Ac, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications
Biotechnological
Sodium acetate is used as the carbon source for culturing bacteria ...
, used in the textile
Textile is an Hyponymy and hypernymy, umbrella term that includes various Fiber, fiber-based materials, including fibers, yarns, Staple (textiles)#Filament fiber, filaments, Thread (yarn), threads, different #Fabric, fabric types, etc. At f ...
industry and as a food preservative
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or b ...
( E262).
*Copper(II) acetate
Copper(II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)2 where AcO− is acetate (). The hydrated derivative, Cu2(OAc)4(H2O)2, which contains one molecule of water for each copper atom, is availab ...
, used as a pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
and a fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality ...
.
* Aluminium acetate and iron(II) acetate
Iron(II) acetate is a coordination complex with formula Fe(O2CCH3)2. It is a white solid, although impure samples can be slightly colored. A light green tetrahydrate is also known, which is highly soluble in water.
Preparation and structure
Ir ...
—used as mordant
A mordant or dye fixative is a substance used to set (i.e. bind) dyes on fabrics by forming a coordination complex with the dye, which then attaches to the fabric (or tissue). It may be used for dyeing fabrics or for intensifying stains in ...
s for dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
s.
* Palladium(II) acetate, used as a catalyst for organic coupling reactions such as the Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
.
Halogenated acetic acids are produced from acetic acid. Some commercially significant derivatives:
*Chloroacetic acid
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds a ...
(monochloroacetic acid, MCA), dichloroacetic acid (considered a by-product), and trichloroacetic acid
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are calle ...
. MCA is used in the manufacture of indigo dye
Indigo dye is an organic compound with a distinctive blue color. Historically, indigo was a natural dye extracted from the leaves of some plants of the ''Indigofera'' genus, in particular '' Indigofera tinctoria''; dye-bearing ''Indigofera'' pl ...
.
*Bromoacetic acid
Bromoacetic acid is the chemical compound with the formula CH2BrCO2H. This colorless solid is a relatively strong alkylating agent. Bromoacetic acid and its esters are widely used building blocks in organic synthesis, for example, in pharmaceuti ...
, which is esterified to produce the reagent ethyl bromoacetate
Ethyl bromoacetate is the chemical compound with the formula CH2BrCO2C2H5. It is the ethyl ester of bromoacetic acid and is prepared in two steps from acetic acid. It is a lachrymator and has a fruity, pungent odor. It is also a highly toxic alk ...
.
*Trifluoroacetic acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with ...
, which is a common reagent in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
.
Amounts of acetic acid used in these other applications together account for another 5–10% of acetic acid use worldwide.
History
Vinegar
Vinegar is an aqueous solution of acetic acid and trace compounds that may include flavorings. Vinegar typically contains 5–8% acetic acid by volume. Usually, the acetic acid is produced by a double fermentation, converting simple sugars to eth ...
was known early in civilization as the natural result of exposure of beer
Beer is one of the oldest and the most widely consumed type of alcoholic drink in the world, and the third most popular drink overall after water and tea. It is produced by the brewing and fermentation of starches, mainly derived from ce ...
and wine
Wine is an alcoholic drink typically made from Fermentation in winemaking, fermented grapes. Yeast in winemaking, Yeast consumes the sugar in the grapes and converts it to ethanol and carbon dioxide, releasing heat in the process. Different ...
to air, because acetic acid-producing bacteria are present globally. The use of acetic acid in alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim wo ...
extends into the 3rd century BC, when the Greek philosopher Theophrastus
Theophrastus (; grc-gre, Θεόφραστος ; c. 371c. 287 BC), a Greek philosopher and the successor to Aristotle in the Peripatetic school. He was a native of Eresos in Lesbos.Gavin Hardy and Laurence Totelin, ''Ancient Botany'', Routle ...
described how vinegar acted on metals to produce pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
s useful in art, including ''white lead'' (lead carbonate
Lead(II) carbonate is the chemical compound with the chemical formula . It is a white solid with several practical uses, despite its toxicity. It occurs naturally as the mineral cerussite.
Structure
Like all metal carbonates, lead(II) carbonate a ...
) and ''verdigris
Verdigris is the common name for blue-green, copper-based pigments that form a patina on copper, bronze, and brass. The technical literature is ambiguous as to its chemical composition. Some sources refer to "neutral verdigris" as copper(II) ...
'', a green mixture of copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
salts including copper(II) acetate
Copper(II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)2 where AcO− is acetate (). The hydrated derivative, Cu2(OAc)4(H2O)2, which contains one molecule of water for each copper atom, is availab ...
. Ancient Romans
Roman or Romans most often refers to:
*Rome, the capital city of Italy
* Ancient Rome, Roman civilization from 8th century BC to 5th century AD
*Roman people, the people of ancient Rome
*''Epistle to the Romans'', shortened to ''Romans'', a lette ...
boiled soured wine to produce a highly sweet syrup called ''sapa''. Sapa
The South African Press Association (SAPA) was the national news agency of South Africa until its closure in 2015.
History
The agency was established on 1 July 1938 by major South African newspapers to facilitate the sharing of news. Reuters had ...
that was produced in lead pots was rich in lead acetate, a sweet substance also called ''sugar of lead'' or ''sugar of Saturn
Saturn is the sixth planet from the Sun and the second-largest in the Solar System, after Jupiter. It is a gas giant with an average radius of about nine and a half times that of Earth. It has only one-eighth the average density of Earth; h ...
'', which contributed to lead poisoning
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. The brain is the most sensitive. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, infertil ...
among the Roman aristocracy.
In the 16th-century German
German(s) may refer to:
* Germany (of or related to)
**Germania (historical use)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizens of Germany, see also German nationality law
**Ge ...
alchemist Andreas Libavius
Andreas Libavius or Andrew Libavius was born in Halle, Germany c. 1550 and died in July 1616. Libavius was a renaissance man who spent time as a professor at the University of Jena teaching history and poetry. After which he became a physician a ...
described the production of acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
from the dry distillation
Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). The method may involve pyrolysis or thermolysis, or it may not (for instance, a simple mixture of ice and glass could be s ...
of lead acetate, ketonic decarboxylation
In organic chemistry, ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid () to a symmetric ketone () by the application of heat w ...
. The presence of water in vinegar has such a profound effect on acetic acid's properties that for centuries chemists believed that glacial acetic acid and the acid found in vinegar were two different substances. French chemist Pierre Adet Pierre-Auguste Adet (17 May 1763 Nevers – 19 March 1834 Paris) was a French scientist, politician, and diplomat.
He worked with Lavoisier on a new chemical notation system, and was secretary to the scientific periodical '' Annales de chimie'', fo ...
proved them identical.
 In 1845 German chemist Hermann Kolbe synthesised acetic acid from
In 1845 German chemist Hermann Kolbe synthesised acetic acid from inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemis ...
s for the first time. This reaction sequence consisted of chlorination Chlorination may refer to:
* Chlorination reaction
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transform ...
of carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
to carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemi ...
, followed by pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements '' ...
to tetrachloroethylene
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2 . It is a colorless li ...
and aqueous chlorination to trichloroacetic acid
Trichloroacetic acid (TCA; TCAA; also known as trichloroethanoic acid) is an analogue of acetic acid in which the three hydrogen atoms of the methyl group have all been replaced by chlorine atoms. Salts and esters of trichloroacetic acid are calle ...
, and concluded with electrolytic reduction to acetic acid.
By 1910, most glacial acetic acid was obtained from the pyroligneous liquor, a product of the distillation of wood. The acetic acid was isolated by treatment with milk of lime, and the resulting calcium acetate was then acidified with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
to recover acetic acid. At that time, Germany was producing 10,000 ton
Ton is the name of any one of several units of measure. It has a long history and has acquired several meanings and uses.
Mainly it describes units of weight. Confusion can arise because ''ton'' can mean
* the long ton, which is 2,240 pounds
...
s of glacial acetic acid, around 30% of which was used for the manufacture of indigo dye
Indigo dye is an organic compound with a distinctive blue color. Historically, indigo was a natural dye extracted from the leaves of some plants of the ''Indigofera'' genus, in particular '' Indigofera tinctoria''; dye-bearing ''Indigofera'' pl ...
.
Because both methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
and carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
are commodity raw materials, methanol carbonylation long appeared to be attractive precursors to acetic acid. Henri Dreyfus
Henri Dreyfus (or Henry Dreyfus, 7 January 1882 – 30 December 1944) was a Swiss inventor of the modern weaving loom. He and his brother Camille Dreyfus also invented Celanese, an acetate yarn.
Early years
Henri Dreyfus was born in 1882, into ...
at British Celanese
British Celanese was a chemical company based in England. Formed in 1916, it survived as an independent company until 1957 when it became a subsidiary of Courtaulds.
History
The origins of the company lie with two brothers, Henri and Camille D ...
developed a methanol carbonylation pilot plant as early as 1925. However, a lack of practical materials that could contain the corrosive reaction mixture at the high pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
s needed (200 atm or more) discouraged commercialization of these routes. The first commercial methanol carbonylation process, which used a cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
catalyst, was developed by German chemical company BASF
BASF SE () is a German multinational chemical company and the largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany.
The BASF Group comprises subsidiaries and joint ventures in more than 80 countries ...
in 1963. In 1968, a rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
-based catalyst (''cis''−) was discovered that could operate efficiently at lower pressure with almost no by-products. US chemical company Monsanto Company
The Monsanto Company () was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best known product is Roundup, a glyphosate-based herbicide, developed i ...
built the first plant using this catalyst in 1970, and rhodium-catalyzed methanol carbonylation became the dominant method of acetic acid production (see Monsanto process). In the late 1990s, the chemicals company BP Chemicals commercialised the Cativa catalyst (), which is promoted by iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density o ...
for greater efficiency. This iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density o ...
-catalyzed Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc. The process is based on an ...
is greener and more efficient and has largely supplanted the Monsanto process, often in the same production plants.
Interstellar medium
Interstellar acetic acid was discovered in 1996 by a team led by David Mehringer using the former Berkeley-Illinois-Maryland Association array at the Hat Creek Radio Observatory and the formerMillimeter Array
The Atacama Large Millimeter/submillimeter Array (ALMA) is an astronomical interferometer of 66 radio telescopes in the Atacama Desert of northern Chile, which observe electromagnetic radiation at millimeter and submillimeter wavelengths. The ...
located at the Owens Valley Radio Observatory. It was first detected in the Sagittarius B2
Sagittarius B2 (Sgr B2) is a giant molecular cloud of gas and dust that is located about from the center of the Milky Way. This complex is the largest molecular cloud in the vicinity of the core and one of the largest in the galaxy, spanning a r ...
North molecular cloud (also known as the Sgr B2 Large Molecule Heimat
The Large Molecule Heimat is a dense gas cloud located in the molecular cloud Sagittarius B2. Many species of molecule, including aminoacetonitrile (a molecule related to glycine), ethyl formate, and butyronitrile
Butyronitrile or butanenitril ...
source). Acetic acid has the distinction of being the first molecule discovered in the interstellar medium using solely radio interferometers; in all previous ISM molecular discoveries made in the millimetre and centimetre wavelength regimes, single dish radio telescopes were at least partly responsible for the detections.
Health effects and safety
Concentrated acetic acid iscorrosive
A corrosive substance is one that will damage or destroy other substances with which it comes into contact by means of a chemical reaction.
Etymology
The word ''corrosive'' is derived from the Latin verb ''corrodere'', which means ''to gnaw'', ...
to skin. These burns or blisters may not appear until hours after exposure.
Prolonged inhalation exposure (eight hours) to acetic acid vapours at 10 ppm can produce some irritation of eyes, nose, and throat; at 100 ppm marked lung irritation and possible damage to lungs, eyes, and skin may result. Vapour concentrations of 1,000 ppm cause marked irritation of eyes, nose and upper respiratory tract and cannot be tolerated. These predictions were based on animal experiments and industrial exposure.
In 12 workers exposed for two or more years to acetic acid airborne average concentration of 51 ppm (estimated), produced symptoms of conjunctive irritation, upper respiratory tract irritation, and hyperkeratotic dermatitis. Exposure to 50 ppm or more is intolerable to most persons and results in intensive lacrimation and irritation of the eyes, nose, and throat, with pharyngeal oedema and chronic bronchitis. Unacclimatised humans experience extreme eye and nasal irritation at concentrations in excess of 25 ppm, and conjunctivitis from concentrations below 10 ppm has been reported. In a study of five workers exposed for seven to 12 years to concentrations of 80 to 200 ppm at peaks, the principal findings were blackening and hyperkeratosis of the skin of the hands, conjunctivitis (but no corneal damage), bronchitis and pharyngitis, and erosion of the exposed teeth (incisors and canines).
The hazards of solutions of acetic acid depend on the concentration. The following table lists the EU classification of acetic acid solutions:
Concentrated acetic acid can be ignited only with difficulty at standard temperature and pressure, but becomes a flammable risk in temperatures greater than , and can form explosive mixtures with air at higher temperatures (explosive limit
Mixtures of dispersed combustible materials (such as gaseous or vaporised fuels, and some dusts) and oxygen in the air will burn only if the fuel concentration lies within well-defined lower and upper bounds determined experimentally, referred to a ...
s: 5.4–16%).
See also
* Acetic acid (data page) *Acids in wine
The acids in wine are an important component in both winemaking and the finished product of wine. They are present in both grapes and wine, having direct influences on the color, balance and taste of the wine as well as the growth and vitality of ...
Notes
References
External links
*National Pollutant Inventory – Acetic acid fact sheet
(US Permissible exposure limits)
*Calculation o
vapor pressure
of acetic acid
Acetic acid bound to proteins
in the PDB
Swedish Chemicals Agency. Information sheet – Acetic Acid
* Process Flow sheet of Acetic acid Production by th
Carbonylation of Methanol
{{portal bar, Medicine Acids in wine Antiseptics Commodity chemicals E-number additives Flavors Household chemicals Otologicals Photographic chemicals Solvents World Health Organization essential medicines Organic compounds with 2 carbon atoms