Ferric chloride on:
[Wikipedia]
[Google]
[Amazon]
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3
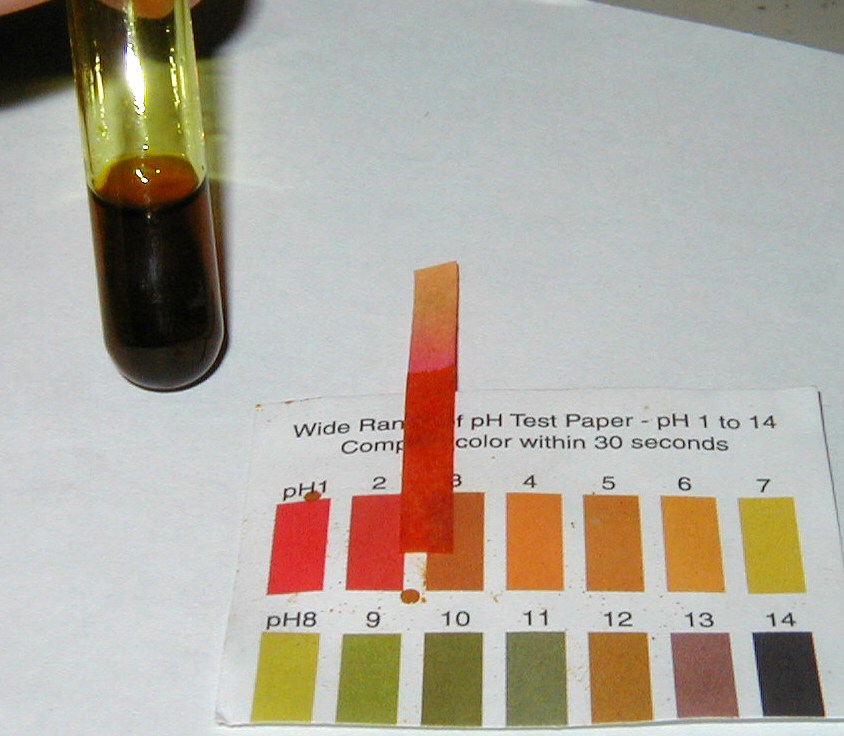 When dissolved in water, iron(III) chloride give a strongly acidic solution.
When heated with iron(III) oxide at 350 °C, iron(III) chloride gives iron oxychloride.
:
The anhydrous salt is a moderately strong Lewis acid, forming
When dissolved in water, iron(III) chloride give a strongly acidic solution.
When heated with iron(III) oxide at 350 °C, iron(III) chloride gives iron oxychloride.
:
The anhydrous salt is a moderately strong Lewis acid, forming
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The colour depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.
Structure and properties
Anhydrous
Anhydrous iron(III) chloride has the structure, withoctahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet a ...
Fe(III) centres interconnected by two-coordinate chloride ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
s.
Iron(III) chloride has a relatively low melting point and boils at around 315 °C. The vapor consists of the dimer (like aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
) which increasingly dissociates into the monomeric
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
(with D3h point group molecular symmetry) at higher temperature, in competition with its reversible decomposition to give iron(II) chloride and chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
gas.
Hydrates
In addition to the anhydrous material, ferric chloride forms four hydrates. All forms of iron(III) chloride feature two or more chlorides as ligands, and three hydrates feature . *''dihydrate'': has the structural formula ''trans''-. * has the structural formula ''cis''-. * has the structural formula ''cis''-. *''hexahydrate'': has the structural formula ''trans''-.Aqueous solution
Aqueous solutions of ferric chloride are characteristically yellow, in contrast to the pale pink solutions of . According to spectroscopic measurements, the main species in aqueous solutions of ferric chloride are the octahedral complex (stereochemistry unspecified) and the tetrahedral .Preparation
Anhydrous iron(III) chloride may be prepared by treating iron with chlorine: : Solutions of iron(III) chloride are produced industrially both from iron and from ore, in a closed-loop process. #Dissolving iron ore inhydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
#:
#Oxidation of iron(II) chloride with chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
#:
#Oxidation of iron(II) chloride with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
and hydrochloric acid
#:
Heating hydrated iron(III) chloride does not yield anhydrous ferric chloride. Instead, the solid decomposes into hydrochloric acid and iron oxychloride. Hydrated iron(III) chloride can be converted to the anhydrous form by treatment with thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year bein ...
. Similarly, dehydration can be effected with trimethylsilyl chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. I ...
:
:
Reactions
adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all co ...
s with Lewis bases such as triphenylphosphine oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in ...
; e.g., where Ph is phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
. It also reacts with other chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride sa ...
salts to give the yellow tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ...
ion. Salts of in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
can be extracted into diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
.
Redox reactions
Iron(III) chloride is a mildoxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
, for example, it oxidizes copper(I) chloride to copper(II) chloride.
:
In a comproportionation reaction, it reacts with iron to form iron(II) chloride:
:
A traditional synthesis of anhydrous ferrous chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as ...
is the reduction of FeCl3 with chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Uses
Historical
The major use of chlorob ...
:
:
With carboxylate anions
Oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
s react rapidly with aqueous iron(III) chloride to give . Other carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylat ...
salts form complexes; e.g., citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the ...
and tartrate
A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. The formula of the tartrate dianion is O−OC-CH(OH)-CH(OH)-COO− or C4H4O62−.
The main forms of tartrates used commercially are pure crystalline ta ...
.
With alkali metal alkoxides
Alkali metal alkoxides react to give the metal alkoxide complexes of varying complexity. The compounds can be dimeric or trimeric. In the solid phase a variety of multinuclear complexes have been described for the nominal stoichiometric reaction between and sodium ethoxide: :With organometallic compounds
Iron(III) chloride inether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
solution oxidizes methyl lithium
Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in s ...
to give first light greenish yellow lithium tetrachloroferrate(III) solution and then, with further addition of methyl lithium, lithium tetrachloroferrate(II) :
:
:
The methyl radical
Methyl (also systematically named trihydridocarbon) is an organic compound with the chemical formula (also written as •). It is a metastable colourless gas, which is mainly produced ''in situ'' as a precursor to other hydrocarbons in the petrol ...
s combine with themselves or react with other components to give mostly ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petroc ...
and some methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
.
Uses
Industrial
Iron(III) chloride is used insewage treatment
Sewage treatment (or domestic wastewater treatment, municipal wastewater treatment) is a type of wastewater treatment which aims to remove contaminants from sewage to produce an effluent that is suitable for discharge to the surrounding e ...
and drinking water production as a coagulant and flocculant. In this application, in slightly basic water reacts with the hydroxide ion () to form a floc of iron(III) hydroxide
Iron(III) oxide-hydroxide or ferric oxyhydroxideA. L. Mackay (1960): "β-Ferric Oxyhydroxide". ''Mineralogical Magazine'' (''Journal of the Mineralogical Society''), volume 32, issue 250, pages 545-557. is the chemical compound of iron, oxygen, ...
(), also formulated as FeO(OH) ( ferrihydrite), that can remove suspended materials.
:
It is also used as a leaching agent in chloride hydrometallurgy, for example in the production of Si from FeSi ( Silgrain process by Elkem).
Another important application of iron(III) chloride is etching copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
in two-step redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
reaction to copper(I) chloride and then to copper(II) chloride in the production of printed circuit boards
A printed circuit board (PCB; also printed wiring board or PWB) is a medium used in electrical and electronic engineering to connect electronic components to one another in a controlled manner. It takes the form of a laminated sandwich struc ...
(PCB).
:
:
Iron(III) chloride is used as catalyst for the reaction of ethylene with chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
, forming ethylene dichloride (1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vin ...
), an important commodity chemical, which is mainly used for the industrial production of vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
, the monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
for making PVC.
:
Laboratory use
In the laboratory iron(III) chloride is commonly employed as a Lewis acid for catalysing reactions such aschlorination Chlorination may refer to:
* Chlorination reaction
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transform ...
of aromatic compounds and Friedel–Crafts reaction of aromatics. It is less powerful than aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
, but in some cases this mildness leads to higher yields, for example in the alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecti ...
of benzene:
:
The ferric chloride test is a traditional colorimetric test for phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are ...
, which uses a 1% iron(III) chloride solution that has been neutralized with sodium hydroxide until a slight precipitate of FeO(OH) is formed. The mixture is filtered before use. The organic substance is dissolved in water, methanol or ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
, then the neutralized iron(III) chloride solution is added—a transient or permanent coloration (usually purple, green or blue) indicates the presence of a phenol or enol.
This reaction is exploited in the Trinder spot test, which is used to indicate the presence of salicylates, particularly salicylic acid, which contains a phenolic OH group.
This test can be used to detect the presence of gamma-hydroxybutyric acid and gamma-butyrolactone, which cause it to turn red-brown.
Other uses
* Used in anhydrous form as a drying reagent in certain reactions. * Used to detect the presence of phenol compounds in organic synthesis; e.g., examining purity of synthesizedAspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat inc ...
.
* Used in water and wastewater treatment to precipitate phosphate as iron(III) phosphate
Iron(III) phosphate, also ferric phosphate, is the inorganic compound with the formula Fe PO4. Several related materials are known, including four polymorphs of FePO4 and two polymorphs of the dihydrate FePO4·(H2O)2. These materials find few ...
.
* Used in wastewater treatment for odor control.
* Used by American coin collectors to identify the dates of Buffalo nickel
The Buffalo nickel or Indian Head nickel is a copper-nickel five-cent piece that was struck by the United States Mint from 1913 to 1938. It was designed by sculptor James Earle Fraser.
As part of a drive to beautify the coinage, five denomin ...
s that are so badly worn that the date is no longer visible.
* Used by bladesmiths and artisans in pattern welding to etch the metal, giving it a contrasting effect, to view metal layering or imperfections.
* Used to etch the widmanstatten pattern in iron meteorites.
* Necessary for the etching of photogravure
Photogravure (in French ''héliogravure'') is a process for printing photographs, also sometimes used for reproductive intaglio printmaking. It is a photo-mechanical process whereby a copper plate is grained (adding a pattern to the plate) and ...
plates for printing photographic and fine art images in intaglio and for etching rotogravure
Rotogravure (or gravure for short) is a type of intaglio printing process, which involves engraving the image onto an image carrier. In gravure printing, the image is engraved onto a cylinder because, like offset printing and flexography, it ...
cylinders used in the printing industry.
* Used to make printed circuit boards (PCBs) by etching copper.
* Used to strip aluminum coating from mirrors.
* Used to etch intricate medical devices.
* Used in veterinary practice to treat overcropping of an animal's claws, particularly when the overcropping results in bleeding.
* Reacts with cyclopentadienylmagnesium bromide in one preparation of ferrocene, a metal-sandwich complex.
* Sometimes used in a technique of Raku ware
is a type of Japanese pottery traditionally used in Japanese tea ceremonies, most often in the form of '' chawan'' tea bowls. It is traditionally characterised by being hand-shaped rather than thrown, fairly porous vessels, which result from low ...
firing, the iron coloring a pottery piece shades of pink, brown, and orange.
* Used to test the pitting and crevice corrosion resistance of stainless steels and other alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s.
* Used in conjunction with NaI in acetonitrile to mildly reduce organic azides to primary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s.
* Used in an animal thrombosis model.
* Used in an experimental energy storage systems.
* Historically it was used to make direct positive blueprint
A blueprint is a reproduction of a technical drawing or engineering drawing using a contact print process on light-sensitive sheets. Introduced by Sir John Herschel in 1842, the process allowed rapid and accurate production of an unlimited number ...
s.
* A component of modified Carnoy's solution used for surgical treatment of keratocystic odontogenic tumor (KOT).
* Used as an additive to sodium chloride (NaCl) to produce clear crystals.
Safety
Iron(III) chloride is harmful, highlycorrosive
A corrosive substance is one that will damage or destroy other substances with which it comes into contact by means of a chemical reaction.
Etymology
The word ''corrosive'' is derived from the Latin verb ''corrodere'', which means ''to gnaw'', ...
and acidic. The anhydrous material is a powerful dehydrating agent.
Although reports of poisoning in humans are rare, ingestion of ferric chloride can result in serious morbidity and mortality. Inappropriate labeling and storage lead to accidental swallowing or misdiagnosis. Early diagnosis is important, especially in seriously poisoned patients.
Natural occurrence
The natural counterpart of is the rare mineral molysite, usually related to volcanic and other-typefumarole
A fumarole (or fumerole) is a vent in the surface of the Earth or other rocky planet from which hot volcanic gases and vapors are emitted, without any accompanying liquids or solids. Fumaroles are characteristic of the late stages of volcani ...
s.
is also produced as an atmospheric salt aerosol by reaction between iron-rich dust and hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
from sea salt. This iron salt aerosol causes about 5% of naturally-occurring oxidization of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
and is thought to have a range of cooling effects.
The atmosphere of the planet Venus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never f ...
is approximately 1% .
See also
*Verhoeff's stain Verhoeff's stain, also known as Verhoeff's elastic stain (VEG) or Verhoeff–Van Gieson stain (VVG), is a staining protocol used in histology, developed by American ophthalmic surgeon and pathologist Frederick Herman Verhoeff (1874–1968) in 1908 ...
*Ferrosilicon
Ferrosilicon is an alloy of iron and silicon with a typical silicon content by weight of 15–90%. It contains a high proportion of iron silicides.
Production and reactions
Ferrosilicon is produced by reduction of silica or sand with coke in t ...
Notes
References
Further reading
# # # # # # {{DEFAULTSORT:Iron(Iii) Chloride Chlorides Iron(III) compounds Metal halides Coordination complexes Deliquescent substances Dehydrating agents Acid catalysts