
Desalination is a process that takes away mineral components from
saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in
soil desalination, which is an issue for agriculture.
Saltwater (especially
sea water
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approx ...
) is desalinated to produce water suitable for
human consumption or
irrigation
Irrigation (also referred to as watering) is the practice of applying controlled amounts of water to land to help grow crops, landscape plants, and lawns. Irrigation has been a key aspect of agriculture for over 5,000 years and has been devel ...
. The
by-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be consid ...
of the desalination process is
brine.
Desalination is used on many seagoing
ship
A ship is a large watercraft that travels the world's oceans and other sufficiently deep waterways, carrying cargo or passengers, or in support of specialized missions, such as defense, research, and fishing. Ships are generally distinguished ...
s and
submarine
A submarine (or sub) is a watercraft capable of independent operation underwater. It differs from a submersible, which has more limited underwater capability. The term is also sometimes used historically or colloquially to refer to remotely op ...
s. Most of the modern interest in desalination is focused on cost-effective provision of
fresh water
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. Although the term specifically excludes seawater and brackish water, it does incl ...
for human use. Along with recycled
wastewater, it is one of the few rainfall-independent
water resources.
Due to its energy consumption, desalinating sea water is generally more costly than fresh water from
surface water
Surface water is water located on top of land forming terrestrial (inland) waterbodies, and may also be referred to as ''blue water'', opposed to the seawater and waterbodies like the ocean.
The vast majority of surface water is produced by pre ...
or
groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit ...
,
water recycling
Water reclamation (also called wastewater reuse, water reuse or water recycling) is the process of converting municipal wastewater (sewage) or industrial wastewater into water that can be reused for a variety of purposes. Types of reuse include: ...
and
water conservation. However, these alternatives are not always available and depletion of reserves is a critical problem worldwide.
Desalination processes are using either thermal methods (in the case of
distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
) or membrane-based methods (e.g. in the case of
reverse osmosis) energy types.
An estimate in 2018 found that "18,426 desalination plants are in operation in over 150 countries. They produce 87 million cubic meters of clean water each day and supply over 300 million people."
The energy intensity has improved: It is now about 3 kWh/m
3 (in 2018), down by a factor of 10 from 20-30 kWh/m
3 in 1970.
Nevertheless, desalination represented about 25% of the energy consumed by the
water sector
The water industry provides drinking water and wastewater services (including sewage treatment) to residential, commercial, and industrial sectors of the economy. Typically public utilities operate water supply networks. The water industry ...
in 2016.
Applications

There are now about 21,000 desalination plants in operation around the globe. The biggest ones are in the
United Arab Emirates
The United Arab Emirates (UAE; ar, اَلْإِمَارَات الْعَرَبِيَة الْمُتَحِدَة ), or simply the Emirates ( ar, الِْإمَارَات ), is a country in Western Asia (Middle East, The Middle East). It is ...
,
Saudi Arabia
Saudi Arabia, officially the Kingdom of Saudi Arabia (KSA), is a country in Western Asia. It covers the bulk of the Arabian Peninsula, and has a land area of about , making it the fifth-largest country in Asia, the second-largest in the Ara ...
, and
Israel
Israel (; he, יִשְׂרָאֵל, ; ar, إِسْرَائِيل, ), officially the State of Israel ( he, מְדִינַת יִשְׂרָאֵל, label=none, translit=Medīnat Yīsrāʾēl; ), is a country in Western Asia. It is situated ...
. The world's largest desalination plant is located in
Saudi Arabia
Saudi Arabia, officially the Kingdom of Saudi Arabia (KSA), is a country in Western Asia. It covers the bulk of the Arabian Peninsula, and has a land area of about , making it the fifth-largest country in Asia, the second-largest in the Ara ...
(
Ras Al-Khair Power and Desalination Plant) with a capacity of 1,401,000 cubic meters per day.
Desalination is currently expensive compared to most alternative sources of water, and only a very small fraction of total human use is satisfied by desalination. It is usually only economically practical for high-valued uses (such as household and industrial uses) in
arid
A region is arid when it severely lacks available water, to the extent of hindering or preventing the growth and development of plant and animal life. Regions with arid climates tend to lack vegetation and are called xeric or desertic. Most ...
areas. However, there is growth in desalination for agricultural use and highly populated areas such as Singapore or California. The most extensive use is in the
Persian Gulf
The Persian Gulf ( fa, خلیج فارس, translit=xalij-e fârs, lit=Gulf of Fars, ), sometimes called the ( ar, اَلْخَلِيْجُ ٱلْعَرَبِيُّ, Al-Khalīj al-ˁArabī), is a mediterranean sea in Western Asia. The bo ...
.
While noting costs are falling, and generally positive about the technology for affluent areas in proximity to oceans, a 2004 study argued, "Desalinated water may be a solution for some water-stress regions, but not for places that are poor, deep in the interior of a continent, or at high elevation. Unfortunately, that includes some of the places with the biggest water problems.", and, "Indeed, one needs to lift the water by 2000 m, or transport it over more than 1600 km to get transport costs equal to the desalination costs.
Thus, it may be more economical to transport fresh water from somewhere else than to desalinate it. In places far from the sea, like
New Delhi
New Delhi (, , ''Naī Dillī'') is the capital of India and a part of the National Capital Territory of Delhi (NCT). New Delhi is the seat of all three branches of the government of India, hosting the Rashtrapati Bhavan, Parliament Hous ...
, or in high places, like
Mexico City
Mexico City ( es, link=no, Ciudad de México, ; abbr.: CDMX; Nahuatl: ''Altepetl Mexico'') is the capital and largest city of Mexico, and the most populous city in North America. One of the world's alpha cities, it is located in the Valley o ...
, transport costs could match desalination costs. Desalinated water is also expensive in places that are both somewhat far from the sea and somewhat high, such as
Riyadh
Riyadh (, ar, الرياض, 'ar-Riyāḍ, lit.: 'The Gardens' Najdi pronunciation: ), formerly known as Hajr al-Yamamah, is the capital and largest city of Saudi Arabia. It is also the capital of the Riyadh Province and the centre of the ...
and
Harare
Harare (; formerly Salisbury ) is the capital and most populous city of Zimbabwe. The city proper has an area of 940 km2 (371 mi2) and a population of 2.12 million in the 2012 census and an estimated 3.12 million in its metropolitan ...
. By contrast in other locations transport costs are much less, such as
Beijing
}
Beijing ( ; ; ), Chinese postal romanization, alternatively romanized as Peking ( ), is the Capital city, capital of the China, People's Republic of China. It is the center of power and development of the country. Beijing is the world's Li ...
,
Bangkok
Bangkok, officially known in Thai as Krung Thep Maha Nakhon and colloquially as Krung Thep, is the capital and most populous city of Thailand. The city occupies in the Chao Phraya River delta in central Thailand and has an estimated populati ...
,
Zaragoza
Zaragoza, also known in English as Saragossa,''Encyclopædia Britannica'"Zaragoza (conventional Saragossa)" is the capital city of the Province of Zaragoza, Zaragoza Province and of the autonomous communities of Spain, autonomous community of Ara ...
,
Phoenix, and, of course, coastal cities like
Tripoli." After desalination at
Jubail
Jubail ( ar, الجبيل, ''Al Jubayl'') is a city in the Eastern province on the Persian Gulf coast of Saudi Arabia, with a total population of 684,531 as of 2021. It is home to the largest industrial city in the world. It is also home to t ...
, Saudi Arabia, water is pumped 320 km inland to
Riyadh
Riyadh (, ar, الرياض, 'ar-Riyāḍ, lit.: 'The Gardens' Najdi pronunciation: ), formerly known as Hajr al-Yamamah, is the capital and largest city of Saudi Arabia. It is also the capital of the Riyadh Province and the centre of the ...
. For coastal cities, desalination is increasingly viewed as a competitive choice.
Not everyone is convinced that desalination is or will be economically viable or environmentally sustainable for the foreseeable future.
Debbie Cook wrote in 2011 that desalination plants can be energy intensive and costly. Therefore, water-stressed regions might do better to focus on conservation or other water supply solutions than invest in desalination plants.
[Desalination: Unlocking Lessons from Yesterday’s Solution (part 1)](_blank)
Water Matters, January 17, 2009.
Desalination provides a method to produce fertilizer from salt, which is removed in desalination process
Technologies
Desalination is an artificial process by which saline water (generally
sea water
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approx ...
) is converted to fresh water. The most common desalination processes are
distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
and
reverse osmosis.
There are several methods. Each has advantages and disadvantages but all are useful. The methods can be divided into membrane-based (e.g.,
reverse osmosis) and thermal-based (e.g.,
multistage flash distillation) methods.
The traditional process of desalination is
distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
(i.e., boiling and re-
condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapo ...
of
seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
to leave salt and impurities behind).
There are currently two technologies with a large majority of the world's desalination capacity:
multi-stage flash distillation and
reverse osmosis.
Distillation
Solar distillation
Solar distillation
A solar still distills water with substances dissolved in it by using the heat of the Sun to evaporate water so that it may be cooled and collected, thereby purifying it. They are used in areas where drinking water is unavailable, so that clea ...
mimics the natural water cycle, in which the sun heats sea water enough for evaporation to occur.
After evaporation, the water vapor is condensed onto a cool surface.
There are two types of solar desalination. The first type uses photovoltaic cells to convert solar energy to electrical energy to power desalination. The second type converts solar energy to heat, is known as solar thermal powered desalination.
Natural evaporation
Water can evaporate through several other physical effects besides solar irradiation. These effects have been included in a multidisciplinary desalination methodology in the
IBTS Greenhouse. The IBTS is an industrial desalination (power)plant on one side and a greenhouse operating with the natural water cycle (scaled down 1:10) on the other side. The various processes of evaporation and condensation are hosted in low-tech utilities, partly underground and the architectural shape of the building itself. This integrated biotectural system is most suitable for large scale
desert greening as it has a km
2 footprint for the water distillation and the same for landscape transformation in desert greening, respectively the regeneration of natural fresh water cycles.
Vacuum distillation
In
vacuum distillation atmospheric pressure is reduced, thus lowering the temperature required to evaporate the water. Liquids boil when the
vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed pha ...
equals the ambient pressure and vapor pressure increases with temperature. Effectively, liquids boil at a lower temperature, when the ambient atmospheric pressure is less than usual atmospheric pressure. Thus, because of the reduced pressure, low-temperature "waste" heat from electrical power generation or industrial processes can be employed.
Multi-stage flash distillation
Water is evaporated and separated from sea water through
multi-stage flash distillation, which is a series of
flash evaporations.
Each subsequent flash process utilizes energy released from the condensation of the water vapor from the previous step.
Multiple-effect distillation
Multiple-effect distillation (MED) works through a series of steps called "effects".
Incoming water is sprayed onto pipes which are then heated to generate steam. The steam is then used to heat the next batch of incoming sea water.
To increase efficiency, the steam used to heat the sea water can be taken from nearby power plants.
Although this method is the most thermodynamically efficient among methods powered by heat,
a few limitations exist such as a max temperature and max number of effects.
Vapor-compression distillation
Vapor-compression evaporation involves using either a mechanical compressor or a jet stream to compress the vapor present above the liquid.
The compressed vapor is then used to provide the heat needed for the evaporation of the rest of the sea water.
Since this system only requires power, it is more cost effective if kept at a small scale.
Wave-powered desalination
Wave powered desalination systems generally convert mechanical wave motion directly to hydraulic power for reverse osmosis.
Such systems aim to maximize efficiency and reduce costs by avoiding conversion to electricity, minimizing excess pressurization above the osmotic pressure, and innovating on hydraulic and wave power components.
One such example is
CETO, a
wave power technology that desalinates seawater using submerged buoys. Wave-powered desalination plants began operating on
Garden Island in Western Australia in 2013 and in
Perth
Perth is the capital and largest city of the Australian state of Western Australia. It is the fourth most populous city in Australia and Oceania, with a population of 2.1 million (80% of the state) living in Greater Perth in 2020. Perth is ...
in 2015.
Membrane distillation
Membrane distillation uses a temperature difference across a membrane to evaporate vapor from a brine solution and condense pure water on the colder side.
The design of the membrane can have a significant effect on efficiency and durability. A study found that a membrane created via co-axial
electrospinning
Electrospinning is a fiber production method that uses electric force to draw charged threads of polymer solutions or polymer melts up to fiber diameters in the order of some hundred nanometers. Electrospinning shares characteristics of both ...
of
PVDF
Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride.
PVDF is a specialty plastic used in applications requiring the highest pu ...
-
HFP and
silica aerogel
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low ...
was able to filter 99.99% of salt after continuous 30 day usage.
Osmosis
Reverse osmosis

The leading process for desalination in terms of installed capacity and yearly growth is
reverse osmosis (RO). The RO membrane processes use semipermeable membranes and applied pressure (on the membrane feed side) to preferentially induce water permeation through the membrane while rejecting salts.
Reverse osmosis plant membrane systems typically use less energy than thermal desalination processes.
Energy cost in desalination processes varies considerably depending on water salinity, plant size and process type. At present the cost of seawater desalination, for example, is higher than traditional water sources, but it is expected that costs will continue to decrease with technology improvements that include, but are not limited to, improved efficiency,
reduction in plant footprint, improvements to plant operation and optimization, more effective feed pretreatment, and lower cost energy sources.
Reverse osmosis uses a thin-film composite membrane, which comprises an ultra-thin, aromatic polyamide thin-film. This polyamide film gives the membrane its transport properties, whereas the remainder of the thin-film composite membrane provides mechanical support. The polyamide film is a dense, void-free polymer with a high surface area, allowing for its high water permeability. A recent study has found that the water permeability is primarily governed by the internal nanoscale mass distribution of the polyamide active layer.
The reverse osmosis process requires maintenance. Various factors interfere with efficiency: ionic contamination (calcium, magnesium etc.);
dissolved organic carbon (DOC); bacteria; viruses;
colloids and insoluble particulates;
biofouling
Biofouling or biological fouling is the accumulation of microorganisms, plants, algae, or small animals where it is not wanted on surfaces such as ship and submarine hulls, devices such as water inlets, pipework, grates, ponds, and rivers tha ...
and
scaling. In extreme cases, the RO membranes are destroyed. To mitigate damage, various pretreatment stages are introduced. Anti-scaling inhibitors include acids and other agents such as the organic polymers
polyacrylamide
Polyacrylamide (abbreviated as PAM) is a polymer with the formula (-CH2CHCONH2-). It has a linear-chain structure. PAM is highly water-absorbent, forming a soft gel when hydrated. In 2008, an estimated 750,000,000 kg were produced, mainly fo ...
and
polymaleic acid,
phosphonates and
polyphosphates. Inhibitors for fouling are
biocide
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a sli ...
s (as oxidants against bacteria and viruses), such as chlorine, ozone, sodium or calcium hypochlorite. At regular intervals, depending on the membrane contamination; fluctuating seawater conditions; or when prompted by monitoring processes, the membranes need to be cleaned, known as emergency or shock-flushing. Flushing is done with inhibitors in a fresh water solution and the system must go offline. This procedure is environmentally risky, since contaminated water is diverted into the ocean without treatment. Sensitive
marine habitats can be irreversibly damaged.
Off-grid
solar-powered desalination unit A solar-powered desalination unit produces potable water from saline water through direct or indirect methods of desalination powered by sunlight. Solar energy is the most promising renewable energy source due to its ability to drive the more po ...
s use solar energy to fill a buffer tank on a hill with seawater. The reverse osmosis process receives its pressurized seawater feed in non-sunlight hours by gravity, resulting in sustainable drinking water production without the need for fossil fuels, an electricity grid or batteries. Nano-tubes are also used for the same function (i.e., Reverse Osmosis).
Forward osmosis
Forward osmosis
Forward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "d ...
uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such as a "draw" solution of high concentration.
Freeze–thaw
Freeze–thaw desalination (or freezing desalination) uses freezing to remove fresh water from salt water. Salt water is sprayed during freezing conditions into a pad where an ice-pile builds up. When seasonal conditions warm, naturally desalinated melt water is recovered. This technique relies on extended periods of natural sub-freezing conditions.
A different freeze–thaw method, not weather dependent and invented by
Alexander Zarchin
Alexander Zarchin (1897–1988) was a Ukrainian-Israeli chemist and inventor. He is most noted for inventing a process of sea water desalination."Jews of Silence." ''B'Or Ha'Torah.'' Number 1, Summer 1982.
Biography
Born in Ukraine to a family ...
, freezes seawater in a vacuum. Under vacuum conditions the ice, desalinated, is melted and diverted for collection and the salt is collected.
Electrodialysis membrane
Electrodialysis utilizes electric potential to move the salts through pairs of charged membranes, which trap salt in alternating channels. Several variances of electrodialysis exist such as conventional
electrodialysis,
electrodialysis reversal
Electrodialysis reversal (EDR) is an electrodialysis reversal water desalination membrane process that has been commercially used since the early 1960s. An electric current migrates dissolved salt ions, including fluorides, nitrates and sulfat ...
.
Microbial desalination
Microbial desalination cells are biological
electrochemical systems that implements the use of electro-active bacteria to power desalination of water
in situ, resourcing the natural anode and cathode gradient of the electro-active bacteria and thus creating an internal
supercapacitor.
Design aspects
Energy consumption
The energy consumption of the desalination process depends on the salinity of the water.
Brackish water desalination requires less energy than
seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
desalination.
The energy intensity of seawater desalination has improved: It is now about 3 kWh/m
3 (in 2018), down by a factor of 10 from 20-30 kWh/m
3 in 1970.
This is similar to the energy consumption of other fresh water supplies transported over large distances, but much higher than local fresh
water supplies
Water supply is the provision of water by public utilities, commercial organisations, community endeavors or by individuals, usually via a system of pumps and pipes. Public water supply systems are crucial to properly functioning societies. Thes ...
that use 0.2 kWh/m
3 or less.
A minimum energy consumption for seawater desalination of around 1 kWh/m
3 has been determined,
excluding prefiltering and intake/outfall pumping. Under 2 kWh/m
3 has been achieved with
reverse osmosis membrane technology, leaving limited scope for further energy reductions as the
reverse osmosis energy consumption in the
1970s was 16 kWh/m
3.
Supplying all US domestic water by desalination would increase domestic
energy consumption by around 10%, about the amount of energy used by domestic refrigerators. Domestic consumption is a relatively small fraction of the total water usage.
Note: "Electrical equivalent" refers to the amount of electrical energy that could be generated using a given quantity of thermal energy and appropriate turbine generator. These calculations do not include the energy required to construct or refurbish items consumed in the process.
Given the energy intensive nature of desalination, with associated economic and environmental costs, desalination is generally considered a last resort after
water conservation. But this is changing as prices continue to fall.
Cogeneration
Cogeneration
Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time.
Cogeneration is a more efficient use of fuel or heat, because otherwise- wasted heat from elec ...
is generating excess heat and electricity generation from a single process. Cogeneration can provide usable heat for desalination in an integrated, or "dual-purpose", facility where a power plant provides the energy for desalination. Alternatively, the facility's energy production may be dedicated to the production of potable water (a stand-alone facility), or excess energy may be produced and incorporated into the energy grid. Cogeneration takes various forms, and theoretically any form of energy production could be used. However, the majority of current and planned cogeneration desalination plants use either
fossil fuels
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels ma ...
or
nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced b ...
as their source of energy. Most plants are located in the
Middle East
The Middle East ( ar, الشرق الأوسط, ISO 233: ) is a geopolitical region commonly encompassing Arabian Peninsula, Arabia (including the Arabian Peninsula and Bahrain), Anatolia, Asia Minor (Asian part of Turkey except Hatay Pro ...
or
North Africa
North Africa, or Northern Africa is a region encompassing the northern portion of the African continent. There is no singularly accepted scope for the region, and it is sometimes defined as stretching from the Atlantic shores of Mauritania in ...
, which use their petroleum resources to offset limited water resources. The advantage of dual-purpose facilities is they can be more efficient in energy consumption, thus making desalination more viable.

The current trend in dual-purpose facilities is hybrid configurations, in which the permeate from reverse osmosis desalination is mixed with distillate from thermal desalination. Basically, two or more desalination processes are combined along with power production. Such facilities have been implemented in Saudi Arabia at
Jeddah and
Yanbu.
A typical
supercarrier in the US military is capable of using nuclear power to desalinate of water per day.
Alternatives to desalination
Increased
water conservation and efficiency remain the most cost-effective approaches in areas with a large potential to improve the efficiency of water use practices. Wastewater reclamation provides multiple benefits over desalination of saline water, although it typically uses desalination membranes.
and storm water capture also provide benefits in treating, restoring and recharging groundwater.
A proposed alternative to desalination in the American Southwest is the commercial importation of bulk water from water-rich areas either by
oil tanker
An oil tanker, also known as a petroleum tanker, is a ship designed for the bulk transport of oil or its products. There are two basic types of oil tankers: crude tankers and product tankers. Crude tankers move large quantities of unrefined ...
s converted to water carriers, or pipelines. The idea is politically unpopular in Canada, where governments imposed trade barriers to bulk water exports as a result of a
North American Free Trade Agreement
The North American Free Trade Agreement (NAFTA ; es, Tratado de Libre Comercio de América del Norte, TLCAN; french: Accord de libre-échange nord-américain, ALÉNA) was an agreement signed by Canada, Mexico, and the United States that crea ...
(NAFTA) claim.
The
California Department of Water Resources and the
California State Water Resources Control Board
The California State Water Resources Control Board (SWRCB) is one of six branches of the California Environmental Protection Agency.
History
This regulatory program has had the status of an official government department since the 1950s. The St ...
submitted a report to the state legislature recommending that urban water suppliers achieve an indoor water use efficiency standard of per capita per day by 2023, declining to per day by 2025, and by 2030 and beyond.
Costs
Factors that determine the costs for desalination include capacity and type of facility, location, feed water, labor, energy, financing and concentrate disposal. Costs of desalinating sea water (infrastructure, energy, and maintenance) are generally higher than fresh water from rivers or
groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit ...
,
water recycling
Water reclamation (also called wastewater reuse, water reuse or water recycling) is the process of converting municipal wastewater (sewage) or industrial wastewater into water that can be reused for a variety of purposes. Types of reuse include: ...
, and
water conservation, but alternatives are not always available. Desalination costs in 2013 ranged from US$0.45 to US$1.00/m
3. More than half of the cost comes directly from energy cost, and since energy prices are very volatile, actual costs can vary substantially.
The cost of untreated fresh water in the developing world can reach US$5/cubic metre.
Desalination
stills control pressure, temperature and brine concentrations to optimize efficiency.
Nuclear-powered desalination might be economical on a large scale.
In 2014, the Israeli facilities of Hadera, Palmahim, Ashkelon, and Sorek were desalinizing water for less than US$0.40 per cubic meter. As of 2006, Singapore was desalinating water for US$0.49 per cubic meter.
Environmental concerns
Intake
In the United States, cooling water intake structures are regulated by the
Environmental Protection Agency (EPA). These structures can have the same impacts on the environment as desalination facility intakes. According to EPA, water intake structures cause adverse environmental impact by sucking fish and shellfish or their eggs into an industrial system. There, the organisms may be killed or injured by heat, physical stress, or chemicals. Larger organisms may be killed or injured when they become trapped against screens at the front of an intake structure. Alternative intake types that mitigate these impacts include beach wells, but they require more energy and higher costs.
The
Kwinana Desalination Plant Kwinana may refer to:
* City of Kwinana, a local government area in Western Australia
* Electoral district of Kwinana, an electorate of the Western Australian Legislative Assembly
* Kwinana Beach, Western Australia, a suburb in Western Australia
* ...
opened in the
Australia
Australia, officially the Commonwealth of Australia, is a sovereign country comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands. With an area of , Australia is the largest country by ...
n city of
Perth
Perth is the capital and largest city of the Australian state of Western Australia. It is the fourth most populous city in Australia and Oceania, with a population of 2.1 million (80% of the state) living in Greater Perth in 2020. Perth is ...
, in 2007. Water there and at
Queensland
)
, nickname = Sunshine State
, image_map = Queensland in Australia.svg
, map_caption = Location of Queensland in Australia
, subdivision_type = Country
, subdivision_name = Australia
, established_title = Before federation
, establishe ...
's
Gold Coast Desalination Plant
The Gold Coast Desalination Plant is a reverse osmosis, water desalination plant located in Bilinga, a seaside suburb of the Gold Coast, in Queensland, Australia. It supplies water to the South East Queensland region via the South East Queen ...
and
Sydney
Sydney ( ) is the capital city of the state of New South Wales, and the most populous city in both Australia and Oceania. Located on Australia's east coast, the metropolis surrounds Sydney Harbour and extends about towards the Blue Mounta ...
's
Kurnell Desalination Plant is withdrawn at , which is slow enough to let fish escape. The plant provides nearly of clean water per day.
[Sullivan, Michael (June 18, 2007]
"Australia Turns to Desalination Amid Water Shortage"
NPR.
Outflow
Desalination processes produce large quantities of
brine, possibly at above ambient temperature, and contain residues of pretreatment and cleaning chemicals, their reaction byproducts and heavy metals due to corrosion (especially in thermal-based plants).
Chemical pretreatment and cleaning are a necessity in most desalination plants, which typically includes prevention of biofouling, scaling, foaming and corrosion in thermal plants, and of biofouling, suspended solids and scale deposits in membrane plants.
To limit the environmental impact of returning the brine to the ocean, it can be diluted with another stream of water entering the ocean, such as the outfall of a
wastewater treatment or power plant. With medium to large power plant and desalination plants, the power plant's cooling water flow is likely to be several times larger than that of the desalination plant, reducing the salinity of the combination. Another method to dilute the brine is to mix it via a diffuser in a mixing zone. For example, once a pipeline containing the brine reaches the sea floor, it can split into many branches, each releasing brine gradually through small holes along its length. Mixing can be combined with power plant or wastewater plant dilution. Furthermore, zero liquid discharge systems can be adopted to treat brine before disposal.
Another possibility is making the desalination plant movable, thus avoiding that the brine builds up into a single location (as it keeps being produced by the desalination plant). Some such movable (ship-connected) desalination plants have been constructed.
Brine is denser than seawater and therefore sinks to the ocean bottom and can damage the ecosystem. Brine plumes have been seen to diminish over time to a diluted concentration, to where there was little to no effect on the surrounding environment. However studies have shown the dilution can be misleading due to the depth at which it occurred. If the dilution was observed during the summer season, there is possibility that there could have been a seasonal thermocline event that could have prevented the concentrated brine to sink to sea floor. This has the potential to not disrupt the sea floor ecosystem and instead the waters above it. Brine dispersal from the desalination plants has been seen to travel several kilometers away, meaning that it has the potential to cause harm to ecosystems far away from the plants. Careful reintroduction with appropriate measures and environmental studies can minimize this problem.
Other issues
Due to the nature of the process, there is a need to place the plants on approximately 25 acres of land on or near the shoreline.
In the case of a plant built inland, pipes have to be laid into the ground to allow for easy intake and outtake.
However, once the pipes are laid into the ground, they have a possibility of leaking into and contaminating nearby aquifers.
Aside from environmental risks, the noise generated by certain types of desalination plants can be loud.
Health aspects
Iodine deficiency
Desalination removes iodine from water and could increase the risk of
iodine deficiency disorders. Israeli researchers claimed a possible link between seawater desalination and iodine deficiency, finding iodine deficits among adults exposed to iodine-poor water concurrently with an increasing proportion of their area's drinking water from seawater reverse osmosis (SWRO). They later found probable iodine deficiency disorders in a population reliant on desalinated seawater.
A possible link of heavy desalinated water use and national iodine deficiency was suggested by Israeli researchers. They found a high burden of iodine deficiency in the general population of Israel: 62% of school-age children and 85% of pregnant women fall below the WHO's adequacy range. They also pointed out the national reliance on iodine-depleted desalinated water, the absence of a universal salt iodization program and reports of increased use of thyroid medication in Israel as a possible reasons that the population's iodine intake is low. In the year that the survey was conducted, the amount of water produced from the desalination plants constitutes about 50% of the quantity of fresh water supplied for all needs and about 80% of the water supplied for domestic and industrial needs in Israel.
Experimental techniques
Other desalination techniques include:
Waste heat
Thermally-driven desalination technologies are frequently suggested for use with low-temperature
waste heat
Waste heat is heat that is produced by a machine, or other process that uses energy, as a byproduct of doing work. All such processes give off some waste heat as a fundamental result of the laws of thermodynamics. Waste heat has lower utilit ...
sources, as the low temperatures are not useful for
process heat needed in many industrial processes, but ideal for the lower temperatures needed for desalination.
In fact, such pairing with waste heat can even improve electrical process:
Diesel generators commonly provide electricity in remote areas. About 40–50% of the energy output is low-grade heat that leaves the engine via the exhaust. Connecting a thermal desalination technology such as
membrane distillation system to the diesel engine exhaust repurposes this low-grade heat for desalination. The system actively cools the
diesel generator, improving its efficiency and increasing its electricity output. This results in an energy-neutral desalination solution. An example plant was commissioned by Dutch company
Aquaver in March 2014 for
Gulhi
Gulhi (Dhivehi: ގުޅި) is one of the inhabited islands of Kaafu Atoll. It is located in the South Malé Atoll close to Maafushi island and connected daily except Friday by MTCC public transport ferry.
Geography
The island is south of the c ...
,
Maldives
Maldives (, ; dv, ދިވެހިރާއްޖެ, translit=Dhivehi Raajje, ), officially the Republic of Maldives ( dv, ދިވެހިރާއްޖޭގެ ޖުމްހޫރިއްޔާ, translit=Dhivehi Raajjeyge Jumhooriyyaa, label=none, ), is an archipelag ...
.
Low-temperature thermal
Originally stemming from
ocean thermal energy conversion research,
low-temperature thermal desalination (LTTD) takes advantage of water boiling at low pressure, even at
ambient temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
. The system uses pumps to create a low-pressure, low-temperature environment in which water boils at a temperature gradient of between two volumes of water. Cool ocean water is supplied from depths of up to . This water is pumped through coils to condense the water vapor. The resulting condensate is purified water. LTTD may take advantage of the temperature gradient available at power plants, where large quantities of warm wastewater are discharged from the plant, reducing the energy input needed to create a temperature gradient.
Experiments were conducted in the US and Japan to test the approach. In Japan, a spray-flash evaporation system was tested by Saga University. In Hawaii, the National Energy Laboratory tested an open-cycle OTEC plant with fresh water and power production using a temperature difference of between surface water and water at a depth of around . LTTD was studied by India's National Institute of Ocean Technology (NIOT) in 2004. Their first LTTD plant opened in 2005 at Kavaratti in the
Lakshadweep
Lakshadweep (), also known as Laccadives (), is a union territory of India. It is an archipelago of 36 islands in the Arabian sea, located off the Malabar Coast.
The name ''Lakshadweep'' means "one lakh islands" in Sanskrit, though the Lac ...
islands. The plant's capacity is /day, at a capital cost of INR 50 million (€922,000). The plant uses deep water at a temperature of .
[Indian Scientists Develop World's First Low Temperature Thermal Desalination Plant]
Retrieved January 1, 2019. In 2007, NIOT opened an experimental, floating LTTD plant off the coast of
Chennai
Chennai (, ), formerly known as Madras ( the official name until 1996), is the capital city of Tamil Nadu, the southernmost Indian state. The largest city of the state in area and population, Chennai is located on the Coromandel Coast of th ...
, with a capacity of /day. A smaller plant was established in 2009 at the North Chennai Thermal Power Station to prove the LTTD application where power plant cooling water is available.
Thermoionic process
In October 2009, Saltworks Technologies announced a process that uses solar or other thermal heat to drive an
ionic current that removes all
sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
and
chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
ions from the water using ion-exchange membranes.
Evaporation and condensation for crops
The
Seawater greenhouse uses natural evaporation and condensation processes inside a
greenhouse
A greenhouse (also called a glasshouse, or, if with sufficient heating, a hothouse) is a structure with walls and roof made chiefly of transparent material, such as glass, in which plants requiring regulated climatic conditions are grown.These ...
powered by solar energy to grow crops in arid coastal land.
Ion concentration polarisation (ICP)
In 2022, using a technique that utilised multiple stages of ion
concentration polarisation followed by a single stage of
electrodialysis, researchers from
MIT manage to create a filterless portable desalination unit, capable of removing both dissolved salts and
suspended solids
Suspended solids refers to small solid particles which remain in suspension in water as a colloid or due to motion of the water. Suspended solids can be removed by sedimentation if their size or density is comparatively large, or by filtration. ...
.
Designed for use by non-experts in remote areas or
natural disaster
A natural disaster is "the negative impact following an actual occurrence of natural hazard in the event that it significantly harms a community". A natural disaster can cause loss of life or damage property, and typically leaves some econ ...
s, as well as on military operations, the prototype is the size of a suitcase, measuring 42 × 33.5 × 19 cm
3 and weighing 9.25 kg.
The process is fully automated, notifying the user when the water is safe to drink, and can be controlled by a single button or smartphone app. As it does not require a high pressure pump the process is highly energy efficient, consuming only 20 watt-hours per liter of drinking water produced, making it capable of being powered by common portable
solar panel
A solar cell panel, solar electric panel, photo-voltaic (PV) module, PV panel or solar panel is an assembly of photovoltaic solar cells mounted in a (usually rectangular) frame, and a neatly organised collection of PV panels is called a photo ...
s. Using a filterless design at low pressures or replacable filters significantly reduces maintenance requirements, while the device itself is self cleaning.
However, the device is limited to producing 0.33 liters of drinking water per minute.
There are also concerns that fouling will impact the long-term reliability, especially in water with high
turbidity. The researchers are working to increase the efficiency and production rate with the intent to commercialise the product in the future, however a significant limitation is the reliance on expensive materials in the current design.
Other approaches
Adsorption-based desalination (AD) relies on the moisture absorption properties of certain materials such as Silica Gel.
Forward osmosis
One process was commercialized by Modern Water PLC using
forward osmosis
Forward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "d ...
, with a number of plants reported to be in operation.
Hydrogel based desalination
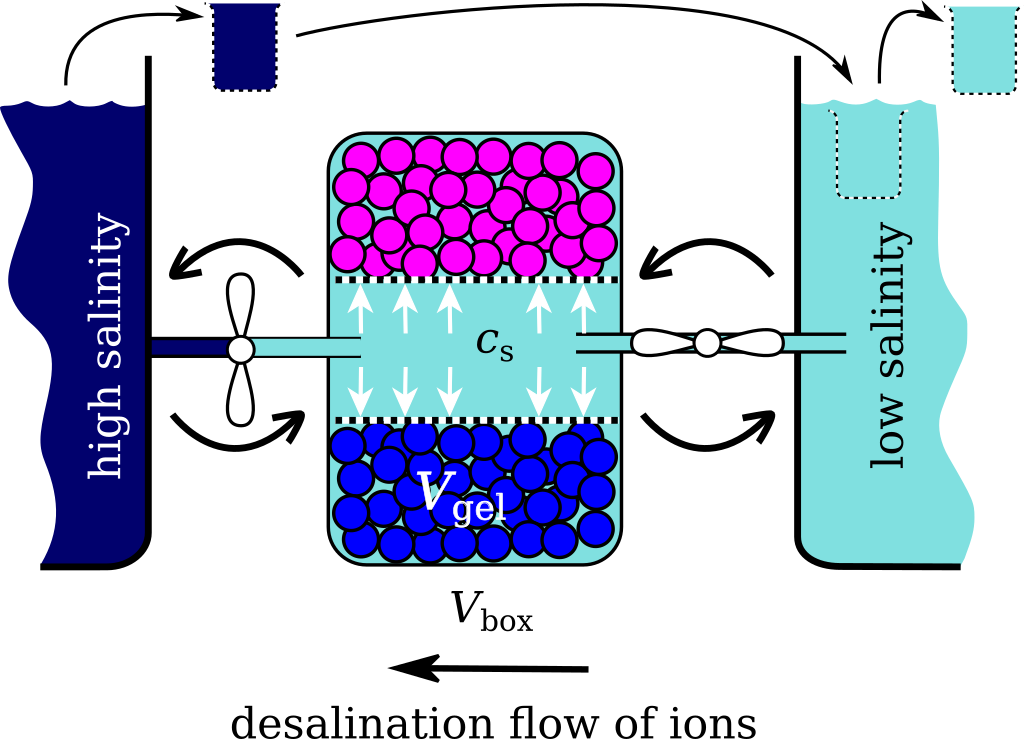
The idea of the method is in the fact that when the hydrogel is put into contact with aqueous salt solution, it swells absorbing a solution with the ion composition different from the original one. This solution can be easily squeezed out from the gel by means of sieve or microfiltration membrane. The compression of the gel in closed system lead to change in salt concentration, whereas the compression in open system, while the gel is exchanging ions with bulk, lead to the change in the number of ions. The consequence of the compression and swelling in open and closed system conditions mimics the reverse Carnot Cycle of refrigerator machine. The only difference is that instead of heat this cycle transfers salt ions from the bulk of low salinity to a bulk of high salinity. Similarly to the Carnot cycle this cycle is fully reversible, so can in principle work with an ideal thermodynamic efficiency. Because the method is free from the use of osmotic membranes it can compete with reverse osmosis method. In addition, unlike the reverse osmosis, the approach is not sensitive to the quality of feed water and its seasonal changes, and allows the production of water of any desired concentration.
Small-scale solar
The United States, France and the United Arab Emirates are working to develop practical
solar desalination
Solar desalination is a desalination technique powered by solar energy. The two common methods are direct (thermal) and indirect (photovoltaic).
History
Solar distillation has been used for thousands of years. Early Greek mariners and Persian al ...
. AquaDania's WaterStillar has been installed at Dahab, Egypt, and in Playa del Carmen, Mexico. In this approach, a
solar thermal collector
A solar thermal collector collects heat by absorbing sunlight. The term "solar collector" commonly refers to a device for solar hot water heating, but may refer to large power generating installations such as solar parabolic troughs and s ...
measuring two square metres can distill from 40 to 60 litres per day from any local water source – five times more than conventional stills. It eliminates the need for plastic
PET bottles or energy-consuming water transport. In Central California, a startup company WaterFX is developing a solar-powered method of desalination that can enable the use of local water, including runoff water that can be treated and used again. Salty groundwater in the region would be treated to become freshwater, and in areas near the ocean, seawater could be treated.
Passarell
The Passarell process uses reduced atmospheric pressure rather than heat to drive evaporative desalination. The pure water vapor generated by distillation is then compressed and condensed using an advanced compressor. The compression process improves distillation efficiency by creating the reduced pressure in the evaporation chamber. The compressor
centrifuge
A centrifuge is a device that uses centrifugal force to separate various components of a fluid. This is achieved by spinning the fluid at high speed within a container, thereby separating fluids of different densities (e.g. cream from milk) or ...
s the pure water vapor after it is drawn through a demister (removing residual impurities) causing it to compress against tubes in the collection chamber. The compression of the vapor increases its temperature. The heat is transferred to the input water falling in the tubes, vaporizing the water in the tubes. Water vapor condenses on the outside of the tubes as product water. By combining several physical processes, Passarell enables most of the system's energy to be recycled through its evaporation, demisting, vapor compression, condensation, and water movement processes.
Geothermal
Geothermal energy can drive desalination. In most locations,
geothermal desalination beats using scarce groundwater or surface water, environmentally and economically.
Nanotechnology
Nanotube membranes of higher permeability than current generation of membranes may lead to eventual reduction in the footprint of RO desalination plants. It has also been suggested that the use of such membranes will lead to reduction in the energy needed for desalination.
Hermetic, sulphonated
nano-composite membranes have shown to be capable of removing various contaminants to the parts per billion level, and have little or no susceptibility to high salt concentration levels.
Biomimesis
Biomimetic membranes
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
are another approach.
Electrochemical
In 2008, Siemens Water Technologies announced technology that applied electric fields to desalinate one cubic meter of water while using only a purported 1.5 kWh of energy. If accurate, this process would consume one-half the energy of other processes. As of 2012 a demonstration plant was operating in Singapore. Researchers at the University of Texas at Austin and the University of Marburg are developing more efficient methods of electrochemically mediated seawater desalination.
Electrokinetic shocks
A process employing electrokinetic shock waves can be used to accomplish membraneless desalination at ambient temperature and pressure. In this process, anions and cations in salt water are exchanged for carbonate anions and calcium cations, respectively using electrokinetic shockwaves. Calcium and carbonate ions react to form
calcium carbonate, which precipitates, leaving fresh water. The theoretical energy efficiency of this method is on par with
electrodialysis and
reverse osmosis.
Temperature swing solvent extraction
Temperature Swing Solvent Extraction (TSSE) uses a solvent instead of a membrane or high temperatures.
Solvent extraction is a common technique in
chemical engineering
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials in ...
. It can be activated by low-grade heat (less than , which may not require active heating. In a study, TSSE removed up to 98.4 percent of the salt in brine. A solvent whose solubility varies with temperature is added to saltwater. At room temperature the solvent draws water molecules away from the salt. The water-laden solvent is then heated, causing the solvent to release the now salt-free water.
It can desalinate extremely salty brine up to seven times as salty as the ocean. For comparison, the current methods can only handle brine twice as salty.
Wave energy
A small-scale offshore system uses wave energy to desalinate 30-50 m
3/day. The system operates with no external power, and is constructed of recycled plastic bottles.
Plants
Trade Arabia claims Saudi Arabia to be producing 7.9 million cubic meters of desalinated water daily, or
22% of world total as of 2021 yearend
*
Perth
Perth is the capital and largest city of the Australian state of Western Australia. It is the fourth most populous city in Australia and Oceania, with a population of 2.1 million (80% of the state) living in Greater Perth in 2020. Perth is ...
began operating a reverse osmosis seawater desalination plant in 2006. The Perth desalination plant is powered partially by renewable energy from the
Emu Downs Wind Farm
The Emu Downs Wind Farm () is a 79.2 MW wind farm in Western Australia. It was a 50:50 joint development between Griffin Energy and Stanwell Corporation. The site is approximately 200 kilometres north of Perth, near Cervantes. Construction of ...
.
* A desalination plant now operates in
Sydney
Sydney ( ) is the capital city of the state of New South Wales, and the most populous city in both Australia and Oceania. Located on Australia's east coast, the metropolis surrounds Sydney Harbour and extends about towards the Blue Mounta ...
, and the
Wonthaggi desalination plant
The Victorian Desalination Plant (also referred to as the Victorian Desalination Project or Wonthaggi desalination plant) is a water desalination plant in Dalyston, on the Bass Coast in southern Victoria, Australia. The project was announced ...
was under construction in
Wonthaggi, Victoria. A wind farm at
Bungendore in
New South Wales
)
, nickname =
, image_map = New South Wales in Australia.svg
, map_caption = Location of New South Wales in AustraliaCoordinates:
, subdivision_type = Country
, subdivision_name = Australia
, established_title = Before federation
, es ...
was purpose-built to generate enough
renewable energy to offset the Sydney plant's energy use, mitigating concerns about harmful
greenhouse gas emissions
Greenhouse gas emissions from human activities strengthen the greenhouse effect, contributing to climate change. Most is carbon dioxide from burning fossil fuels: coal, oil, and natural gas. The largest emitters include coal in China and ...
.
* A January 17, 2008, article in ''
The Wall Street Journal
''The Wall Street Journal'' is an American business-focused, international daily newspaper based in New York City, with international editions also available in Chinese and Japanese. The ''Journal'', along with its Asian editions, is published ...
'' stated, "In November, Connecticut-based Poseidon Resources Corp. won a key regulatory approval to build the $300 million water-
desalination plant in
Carlsbad, north of
San Diego
San Diego ( , ; ) is a city on the Pacific Ocean coast of Southern California located immediately adjacent to the Mexico–United States border. With a 2020 population of 1,386,932, it is the eighth most populous city in the United States ...
. The facility would produce 190,000 cubic metres of drinking water per day, enough to supply about 100,000 homes. As of June 2012, the cost for the desalinated water had risen to $2,329 per acre-foot. Each $1,000 per acre-foot works out to $3.06 for 1,000 gallons, or $0.81 per cubic meter.
As new technological innovations continue to reduce the capital cost of desalination, more countries are building desalination plants as a small element in addressing their
water scarcity
Water scarcity (closely related to water stress or water crisis) is the lack of fresh water resources to meet the standard water demand. There are two types of water scarcity: physical or economic water scarcity. Physical water scarcity is whe ...
problems.
*
Israel
Israel (; he, יִשְׂרָאֵל, ; ar, إِسْرَائِيل, ), officially the State of Israel ( he, מְדִינַת יִשְׂרָאֵל, label=none, translit=Medīnat Yīsrāʾēl; ), is a country in Western Asia. It is situated ...
desalinizes water for a cost of 53 cents per cubic meter
*
Singapore
Singapore (), officially the Republic of Singapore, is a sovereign island country and city-state in maritime Southeast Asia. It lies about one degree of latitude () north of the equator, off the southern tip of the Malay Peninsula, bor ...
desalinizes water for 49 cents per cubic meter and also treats sewage with
reverse osmosis for industrial and potable use (
NEWater
NEWater is the brand name given to highly treated reclaimed wastewater produced by Singapore's Public Utilities Board. NEWater is produced by further purifying conventionally treated wastewater through microfiltration, reverse osmosis and ul ...
).
* China and India, the world's two most populous countries, are turning to desalination to provide a small part of their water needs
* In 2007 Pakistan announced plans to use desalination
* All Australian capital cities (except
Canberra,
Darwin, Northern Territory and
Hobart
Hobart ( ; Nuennonne/ Palawa kani: ''nipaluna'') is the capital and most populous city of the Australian island state of Tasmania. Home to almost half of all Tasmanians, it is the least-populated Australian state capital city, and second-small ...
) are either in the process of building desalination plants, or are already using them. In late 2011,
Melbourne
Melbourne ( ; Boonwurrung/ Woiwurrung: ''Narrm'' or ''Naarm'') is the capital and most populous city of the Australian state of Victoria, and the second-most populous city in both Australia and Oceania. Its name generally refers to a metro ...
will begin using Australia's largest desalination plant, the
Wonthaggi desalination plant
The Victorian Desalination Plant (also referred to as the Victorian Desalination Project or Wonthaggi desalination plant) is a water desalination plant in Dalyston, on the Bass Coast in southern Victoria, Australia. The project was announced ...
to raise low reservoir levels.
* In 2007
Bermuda
)
, anthem = "God Save the King"
, song_type = National song
, song = "Hail to Bermuda"
, image_map =
, map_caption =
, image_map2 =
, mapsize2 =
, map_caption2 =
, subdivision_type = Sovereign state
, subdivision_name =
, es ...
signed a contract to purchase a desalination plant
* The largest desalination plant in the
United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country Continental United States, primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., ...
is at
Tampa Bay,
Florida
Florida is a state located in the Southeastern region of the United States. Florida is bordered to the west by the Gulf of Mexico, to the northwest by Alabama, to the north by Georgia, to the east by the Bahamas and Atlantic Ocean, and ...
, which began desalinizing 25 million gallons (95000 m³) of water per day in December 2007. In the United States, the cost of desalination is $3.06 for 1,000 gallons, or 81 cents per cubic meter. In the United States,
California
California is a state in the Western United States, located along the Pacific Coast. With nearly 39.2million residents across a total area of approximately , it is the most populous U.S. state and the 3rd largest by area. It is also the m ...
,
Arizona
Arizona ( ; nv, Hoozdo Hahoodzo ; ood, Alĭ ṣonak ) is a state in the Southwestern United States. It is the 6th largest and the 14th most populous of the 50 states. Its capital and largest city is Phoenix. Arizona is part of the Fou ...
,
Texas
Texas (, ; Spanish: ''Texas'', ''Tejas'') is a state in the South Central region of the United States. At 268,596 square miles (695,662 km2), and with more than 29.1 million residents in 2020, it is the second-largest U.S. state by ...
, and Florida use desalination for a very small part of their water supply.
* After being desalinized at
Jubail
Jubail ( ar, الجبيل, ''Al Jubayl'') is a city in the Eastern province on the Persian Gulf coast of Saudi Arabia, with a total population of 684,531 as of 2021. It is home to the largest industrial city in the world. It is also home to t ...
,
Saudi Arabia
Saudi Arabia, officially the Kingdom of Saudi Arabia (KSA), is a country in Western Asia. It covers the bulk of the Arabian Peninsula, and has a land area of about , making it the fifth-largest country in Asia, the second-largest in the Ara ...
, water is pumped inland though a pipeline to the capital city of
Riyadh
Riyadh (, ar, الرياض, 'ar-Riyāḍ, lit.: 'The Gardens' Najdi pronunciation: ), formerly known as Hajr al-Yamamah, is the capital and largest city of Saudi Arabia. It is also the capital of the Riyadh Province and the centre of the ...
.
As of 2008, "World-wide, 13,080 desalination plants produce more than 12 billion gallons of water a day, according to the International Desalination Association." An estimate in 2009 found that the worldwide desalinated water supply will triple between 2008 and 2020.
One of the world's largest desalination hubs is the
Jebel Ali Power Generation and Water Production Complex in the
United Arab Emirates
The United Arab Emirates (UAE; ar, اَلْإِمَارَات الْعَرَبِيَة الْمُتَحِدَة ), or simply the Emirates ( ar, الِْإمَارَات ), is a country in Western Asia (Middle East, The Middle East). It is ...
. It is a site featuring multiple plants using different desalination technologies and is capable of producing 2.2 million cubic meters of water per day.
A typical
aircraft carrier in the U.S. military uses nuclear power to desalinize of water per day.
In nature

Evaporation of water over the oceans in the
water cycle
The water cycle, also known as the hydrologic cycle or the hydrological cycle, is a biogeochemical cycle that describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly co ...
is a natural desalination process.
The formation of
sea ice produces ice with little salt, much lower than in seawater.
Seabirds distill seawater using
countercurrent exchange
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some chemical, between two flowing bodies flowing in opposite directions to each ...
in a
gland
In animals, a gland is a group of cells in an animal's body that synthesizes substances (such as hormones) for release into the bloodstream (endocrine gland) or into cavities inside the body or its outer surface (exocrine gland).
Structure
De ...
with a
rete mirabile. The gland
secretes highly concentrated brine stored near the nostrils above the beak. The bird then "sneezes" the brine out. As freshwater is not usually available in their environments, some seabirds, such as
pelicans,
petrels,
albatross
Albatrosses, of the biological family Diomedeidae, are large seabirds related to the procellariids, storm petrels, and diving petrels in the order Procellariiformes (the tubenoses). They range widely in the Southern Ocean and the North Pac ...
es,
gull
Gulls, or colloquially seagulls, are seabirds of the family Laridae in the suborder Lari. They are most closely related to the terns and skimmers and only distantly related to auks, and even more distantly to waders. Until the 21st century ...
s and
terns, possess this gland, which allows them to drink the salty water from their environments while they are far from land.
Mangrove trees grow in seawater; they secrete salt by trapping it in parts of the root, which are then eaten by animals (usually crabs). Additional salt is removed by storing it in leaves that fall off. Some types of mangroves have glands on their leaves, which work in a similar way to the seabird desalination gland. Salt is extracted to the leaf exterior as small
crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
s, which then fall off the leaf.
Willow trees and
reeds absorb salt and other contaminants, effectively desalinating the water. This is used in artificial
constructed wetlands, for treating
sewage.
History
Desalination has been known to history for millennia as both a concept, and later practice, though in a limited form. The ancient Greek philosopher
Aristotle
Aristotle (; grc-gre, Ἀριστοτέλης ''Aristotélēs'', ; 384–322 BC) was a Greek philosopher and polymath during the Classical period in Ancient Greece. Taught by Plato, he was the founder of the Peripatetic school of ...
observed in his work ''
Meteorology
Meteorology is a branch of the atmospheric sciences (which include atmospheric chemistry and physics) with a major focus on weather forecasting. The study of meteorology dates back millennia, though significant progress in meteorology did no ...
'' that "salt water, when it turns into vapour, becomes sweet and the vapour does not form salt water again when it condenses," and also noticed that a fine wax vessel would hold potable water after being submerged long enough in seawater, having acted as a membrane to filter the salt. There are numerous other examples of experimentation in desalination throughout Antiquity and the Middle Ages, but desalination was never feasible on a large scale until the modern era. A good example of this experimentation are the observations by
Leonardo da Vinci
Leonardo di ser Piero da Vinci (15 April 14522 May 1519) was an Italian polymath of the High Renaissance who was active as a painter, draughtsman, engineer, scientist, theorist, sculptor, and architect. While his fame initially rested on ...
(Florence, 1452), who realized that distilled water could be made cheaply in large quantities by adapting a
still to a cookstove. During the Middle Ages elsewhere in Central Europe, work continued on refinements in distillation, although not necessarily directed towards desalination.
However, it is possible that the first major land-based desalination plant may have been installed under emergency conditions on an island off the coast of Tunisia in 1560.
It is believed that a garrison of 700 Spanish soldiers was besieged by a large number of Turks and that, during the siege, the captain in charge fabricated a
still capable of producing 40 barrels of fresh water per day, though details of the device have not been reported.
Before the
Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going f ...
, desalination was primarily of concern to oceangoing ships, which otherwise needed to keep on board supplies of fresh water. Sir
Richard Hawkins (1562-1622), who made extensive travels in the South Seas, reported in his return that he had been able to supply his men with fresh water by means of shipboard distillation. Additionally, during the early 1600s, several prominent figures of the era such as
Francis Bacon
Francis Bacon, 1st Viscount St Alban (; 22 January 1561 – 9 April 1626), also known as Lord Verulam, was an English philosopher and statesman who served as Attorney General and Lord Chancellor of England. Bacon led the advancement of both ...
or
Walter Raleigh
Sir Walter Raleigh (; – 29 October 1618) was an English statesman, soldier, writer and explorer. One of the most notable figures of the Elizabethan era, he played a leading part in English colonisation of North America, suppressed rebelli ...
published reports on water desalination.
These reports and others, set the climate for the first patent dispute concerning desalination apparatus. The two first patents regarding water desalination date back to 1675 and 1683 (patents No.184 and No. 226, published by Mr. William Walcot and Mr. Robert Fitzgerald (and others), respectively). Nevertheless, neither of the two inventions was really put into service as a consequence of technical problems derived from scale-up difficulties.
No significant improvements to the basic seawater distillation process were made for some time during the 150 years from mid-1600s until 1800.
When the frigate ''
Protector'' was sold to Denmark in the 1780s (as the ship ''Hussaren'') the desalination plant was studied and recorded in great detail. In the newly formed United States, Thomas Jefferson catalogued heat-based methods going back to the 1500s, and formulated practical advice that was publicized to all U.S. ships on the backs of sailing clearance permits.
Beginning about 1800, things started changing very rapidly as consequence of the appearance of the
steam engine
A steam engine is a heat engine that performs mechanical work using steam as its working fluid. The steam engine uses the force produced by steam pressure to push a piston back and forth inside a cylinder. This pushing force can be ...
and the so-called
age of steam.
The development of a knowledge of the thermodynamics of steam processes and the need for a pure water source for its use in boilers, generated a positive effect regarding distilling systems. Additionally, the spread of
European colonialism induced a need for freshwater in remote parts of the world, thus creating the appropriate climate for water desalination.
In parallel with the development and improvement of systems using steam (
multiple-effect evaporators), this type of devices quickly demonstrated their potential in the field of desalination.
In 1852,
Alphonse René le Mire de Normandy, was issued a British patent for a vertical tube seawater distilling unit which thanks to its simplicity of design and ease of construction, very quickly gained popularity for shipboard use.
[James D. Birkett. History, development and management of water resources – Vol. I. The history of desalination before large-scale use. EOLSS Publications, (2010).] Land-based desalting units did not significantly appear until the later half of the nineteenth century.
In the 1860s, the US Army purchased three Normandy evaporators, each rated at 7000 gallons/day and installed them on the islands of
Key West
Key West ( es, Cayo Hueso) is an island in the Straits of Florida, within the U.S. state of Florida. Together with all or parts of the separate islands of Dredgers Key, Fleming Key, Sunset Key, and the northern part of Stock Island, it cons ...
and
Dry Tortugas.
Another important land-based desalter plant was installed at
Suakin during the 1880s which was able to provide freshwater to the British troops placed there. It consisted of six-effect distillers with a capacity of 350 tons/day.
Significant research into improved desalination methods occurred in the United States after World War II. The Office of Saline Water was created in the
United States Department of the Interior
The United States Department of the Interior (DOI) is one of the executive departments of the U.S. federal government headquartered at the Main Interior Building, located at 1849 C Street NW in Washington, D.C. It is responsible for the ma ...
in 1955 in accordance with the Saline Water Conversion Act of 1952.
It was merged into the Office of Water Resources Research in 1974.
The first industrial desalination plant in the United States opened in
Freeport, Texas in 1961 with the hope of bringing
water security to the region after a decade of drought.
Vice-president
Lyndon B. Johnson attended the plant's opening on June 21, 1961. President
John F. Kennedy recorded a speech from the
White House
The White House is the official residence and workplace of the president of the United States. It is located at 1600 Pennsylvania Avenue NW in Washington, D.C., and has been the residence of every U.S. president since John Adams in ...
, describing desalination as "a work that in many ways is more important than any other scientific enterprise in which this country is now engaged."
Research took place at state universities in California, at the
Dow Chemical Company and
DuPont
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in ...
.
Many studies focus on ways to optimize desalination systems.
The first commercial
reverse osmosis desalination plant, Coalinga desalination plant, was inaugurated in
California
California is a state in the Western United States, located along the Pacific Coast. With nearly 39.2million residents across a total area of approximately , it is the most populous U.S. state and the 3rd largest by area. It is also the m ...
in 1965 for
brackish water. A few years later, in 1975, the first
sea water
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approx ...
reverse osmosis desalination plant came into operation.
Society and culture
Despite the issues associated with desalination processes, public support for its development can be very high.
One survey of a Southern California community saw 71.9% of all respondents being in support of desalination plant development in their community.
In many cases, high freshwater scarcity corresponds to higher public support for desalination development whereas areas with low water scarcity tend to have less public support for its development.
See also
*
Atmospheric water generator
An atmospheric water generator (AWG), is a device that extracts water from humid ambient air, producing potable water. Water vapor in the air can be extracted by multiple techniques, including condensation - cooling the air below its dew point, e ...
*
Dewvaporation
*
Flexible barge
*
Peak water
*
Pumpable ice technology
*
Soil desalination model
*
Soil salinity
Soil salinity is the salt (chemistry), salt content in the soil; the process of increasing the salt content is known as salinization. Salts occur naturally within soils and water. Salination can be caused by natural processes such as mineral wea ...
*
Soil salinity and groundwater model
References
External links
International Desalination AssociationEuropean Desalination SocietyWorking principles in desalination systemsClassification of Desalination Technologies (CDT)SOLAR TOWER Project – Clean Electricity Generation for Desalination.Encyclopedia of Desalination and water and Water Resources
{{Authority control
Environmental issues with water
Filters
Fresh water
Water supply
Water desalination
Water treatment
 The leading process for desalination in terms of installed capacity and yearly growth is reverse osmosis (RO). The RO membrane processes use semipermeable membranes and applied pressure (on the membrane feed side) to preferentially induce water permeation through the membrane while rejecting salts. Reverse osmosis plant membrane systems typically use less energy than thermal desalination processes. Energy cost in desalination processes varies considerably depending on water salinity, plant size and process type. At present the cost of seawater desalination, for example, is higher than traditional water sources, but it is expected that costs will continue to decrease with technology improvements that include, but are not limited to, improved efficiency, reduction in plant footprint, improvements to plant operation and optimization, more effective feed pretreatment, and lower cost energy sources.
Reverse osmosis uses a thin-film composite membrane, which comprises an ultra-thin, aromatic polyamide thin-film. This polyamide film gives the membrane its transport properties, whereas the remainder of the thin-film composite membrane provides mechanical support. The polyamide film is a dense, void-free polymer with a high surface area, allowing for its high water permeability. A recent study has found that the water permeability is primarily governed by the internal nanoscale mass distribution of the polyamide active layer.
The reverse osmosis process requires maintenance. Various factors interfere with efficiency: ionic contamination (calcium, magnesium etc.); dissolved organic carbon (DOC); bacteria; viruses; colloids and insoluble particulates;
The leading process for desalination in terms of installed capacity and yearly growth is reverse osmosis (RO). The RO membrane processes use semipermeable membranes and applied pressure (on the membrane feed side) to preferentially induce water permeation through the membrane while rejecting salts. Reverse osmosis plant membrane systems typically use less energy than thermal desalination processes. Energy cost in desalination processes varies considerably depending on water salinity, plant size and process type. At present the cost of seawater desalination, for example, is higher than traditional water sources, but it is expected that costs will continue to decrease with technology improvements that include, but are not limited to, improved efficiency, reduction in plant footprint, improvements to plant operation and optimization, more effective feed pretreatment, and lower cost energy sources.
Reverse osmosis uses a thin-film composite membrane, which comprises an ultra-thin, aromatic polyamide thin-film. This polyamide film gives the membrane its transport properties, whereas the remainder of the thin-film composite membrane provides mechanical support. The polyamide film is a dense, void-free polymer with a high surface area, allowing for its high water permeability. A recent study has found that the water permeability is primarily governed by the internal nanoscale mass distribution of the polyamide active layer.
The reverse osmosis process requires maintenance. Various factors interfere with efficiency: ionic contamination (calcium, magnesium etc.); dissolved organic carbon (DOC); bacteria; viruses; colloids and insoluble particulates;  The current trend in dual-purpose facilities is hybrid configurations, in which the permeate from reverse osmosis desalination is mixed with distillate from thermal desalination. Basically, two or more desalination processes are combined along with power production. Such facilities have been implemented in Saudi Arabia at Jeddah and Yanbu.
A typical supercarrier in the US military is capable of using nuclear power to desalinate of water per day.
The current trend in dual-purpose facilities is hybrid configurations, in which the permeate from reverse osmosis desalination is mixed with distillate from thermal desalination. Basically, two or more desalination processes are combined along with power production. Such facilities have been implemented in Saudi Arabia at Jeddah and Yanbu.
A typical supercarrier in the US military is capable of using nuclear power to desalinate of water per day.
 The idea of the method is in the fact that when the hydrogel is put into contact with aqueous salt solution, it swells absorbing a solution with the ion composition different from the original one. This solution can be easily squeezed out from the gel by means of sieve or microfiltration membrane. The compression of the gel in closed system lead to change in salt concentration, whereas the compression in open system, while the gel is exchanging ions with bulk, lead to the change in the number of ions. The consequence of the compression and swelling in open and closed system conditions mimics the reverse Carnot Cycle of refrigerator machine. The only difference is that instead of heat this cycle transfers salt ions from the bulk of low salinity to a bulk of high salinity. Similarly to the Carnot cycle this cycle is fully reversible, so can in principle work with an ideal thermodynamic efficiency. Because the method is free from the use of osmotic membranes it can compete with reverse osmosis method. In addition, unlike the reverse osmosis, the approach is not sensitive to the quality of feed water and its seasonal changes, and allows the production of water of any desired concentration.
The idea of the method is in the fact that when the hydrogel is put into contact with aqueous salt solution, it swells absorbing a solution with the ion composition different from the original one. This solution can be easily squeezed out from the gel by means of sieve or microfiltration membrane. The compression of the gel in closed system lead to change in salt concentration, whereas the compression in open system, while the gel is exchanging ions with bulk, lead to the change in the number of ions. The consequence of the compression and swelling in open and closed system conditions mimics the reverse Carnot Cycle of refrigerator machine. The only difference is that instead of heat this cycle transfers salt ions from the bulk of low salinity to a bulk of high salinity. Similarly to the Carnot cycle this cycle is fully reversible, so can in principle work with an ideal thermodynamic efficiency. Because the method is free from the use of osmotic membranes it can compete with reverse osmosis method. In addition, unlike the reverse osmosis, the approach is not sensitive to the quality of feed water and its seasonal changes, and allows the production of water of any desired concentration.