covalency on:
[Wikipedia]
[Google]
[Amazon]
 A covalent bond is a
A covalent bond is a
 The term ''covalence'' in regard to bonding was first used in 1919 by Irving Langmuir in a ''
The term ''covalence'' in regard to bonding was first used in 1919 by Irving Langmuir in a ''
 Bonds with one or three electrons can be found in radical species, which have an odd number of electrons. The simplest example of a 1-electron bond is found in the
Bonds with one or three electrons can be found in radical species, which have an odd number of electrons. The simplest example of a 1-electron bond is found in the
Covalent Bonds and Molecular Structure
{{Authority control Chemical bonding
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
that involves the sharing of electrons to form electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper he ...
s between atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s, is known as covalent bonding. For many molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
s, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
.
Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interaction
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, r ...
s, bent bond
In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a ...
s, three-center two-electron bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''-b ...
s and three-center four-electron bond
The 3-center 4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur tetrafluoride, the xenon fluorides, and the bifluoride ion. It is also know ...
s. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such as is discussed in valence bond theory.
In the molecule , the hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atoms share the two electrons via covalent bonding. Covalency is greatest between atoms of similar electronegativities
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
. Thus, covalent bonding does not necessarily require that the two atoms be of the same elements, only that they be of comparable electronegativity. Covalent bonding that entails the sharing of electrons over more than two atoms is said to be delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
.
History
Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytic ...
'' article entitled "The Arrangement of Electrons in Atoms and Molecules". Langmuir wrote that "we shall denote by the term ''covalence'' the number of pairs of electrons that a given atom shares with its neighbors."
The idea of covalent bonding can be traced several years before 1919 to Gilbert N. Lewis, who in 1916 described the sharing of electron pairs between atoms. He introduced the '' Lewis notation'' or ''electron dot notation'' or ''Lewis dot structure'', in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. Pairs of electrons located between atoms represent covalent bonds. Multiple pairs represent multiple bonds, such as double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
s and triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
s. An alternative form of representation, not shown here, has bond-forming electron pairs represented as solid lines.
Lewis proposed that an atom forms enough covalent bonds to form a full (or closed) outer electron shell. In the diagram of methane shown here, the carbon atom has a valence of four and is, therefore, surrounded by eight electrons (the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rul ...
), four from the carbon itself and four from the hydrogens bonded to it. Each hydrogen has a valence of one and is surrounded by two electrons (a duet rule) – its own one electron plus one from the carbon. The numbers of electrons correspond to full shells in the quantum theory of the atom; the outer shell of a carbon atom is the ''n'' = 2 shell, which can hold eight electrons, whereas the outer (and only) shell of a hydrogen atom is the ''n'' = 1 shell, which can hold only two.
While the idea of shared electron pairs provides an effective qualitative picture of covalent bonding, quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistr ...
is needed to understand the nature of these bonds and predict the structures and properties of simple molecules. Walter Heitler
Walter Heinrich Heitler (; 2 January 1904 – 15 November 1981) was a German physicist who made contributions to quantum electrodynamics and quantum field theory. He brought chemistry under quantum mechanics through his theory of valence bond ...
and Fritz London
Fritz Wolfgang London (March 7, 1900 – March 30, 1954) was a German physicist and professor at Duke University. His fundamental contributions to the theories of chemical bonding and of intermolecular forces ( London dispersion forces) are today ...
are credited with the first successful quantum mechanical explanation of a chemical bond (molecular hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, ...
) in 1927. English translation in Their work was based on the valence bond model, which assumes that a chemical bond is formed when there is good overlap between the atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
of participating atoms.
Types of covalent bonds
Atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
(except for s orbitals) have specific directional properties leading to different types of covalent bonds. Sigma (σ) bonds are the strongest covalent bonds and are due to head-on overlapping of orbitals on two different atoms. A single bond is usually a σ bond. Pi (π) bonds are weaker and are due to lateral overlap between p (or d) orbitals. A double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
between two given atoms consists of one σ and one π bond, and a triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
is one σ and two π bonds.
Covalent bonds are also affected by the electronegativity of the connected atoms which determines the chemical polarity of the bond. Two atoms with equal electronegativity will make nonpolar covalent bonds such as H–H. An unequal relationship creates a polar covalent bond such as with H−Cl. However polarity also requires geometric asymmetry, or else dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system ...
s may cancel out, resulting in a non-polar molecule.
Covalent structures
There are several types of structures for covalent substances, including individual molecules,molecular structures
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that deter ...
, macromolecular
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
structures and giant covalent structures. Individual molecules have strong bonds that hold the atoms together, but generally, there are negligible forces of attraction between molecules. Such covalent substances are usually gases, for example, HCl, SO2, CO2, and CH4. In molecular structures, there are weak forces of attraction. Such covalent substances are low-boiling-temperature liquids (such as ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
), and low-melting-temperature solids (such as iodine and solid CO2). Macromolecular structures have large numbers of atoms linked by covalent bonds in chains, including synthetic polymers such as polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including b ...
and nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pe ...
, and biopolymers such as protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s and starch. Network covalent structures (or giant covalent structures) contain large numbers of atoms linked in sheets (such as graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on lar ...
), or 3-dimensional structures (such as diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, ...
and quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
). These substances have high melting and boiling points, are frequently brittle, and tend to have high electrical resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
. Elements that have high electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
, and the ability to form three or four electron pair bonds, often form such large macromolecular structures.
One- and three-electron bonds
dihydrogen cation
The dihydrogen cation or hydrogen molecular ion is a cation (positive ion) with formula . It consists of two hydrogen nuclei ( protons) sharing a single electron. It is the simplest molecular ion.
The ion can be formed from the ionization of a ...
, . One-electron bonds often have about half the bond energy of a 2-electron bond, and are therefore called "half bonds". However, there are exceptions: in the case of dilithium
Dilithium, Li2, is a strongly electrophilic, diatomic molecule comprising two lithium atoms covalently bonded together. Li2 is known in the gas phase.
It has a bond order of 1, an internuclear separation of 267.3 pm and a bond energy o ...
, the bond is actually stronger for the 1-electron than for the 2-electron Li2. This exception can be explained in terms of hybridization and inner-shell effects.
The simplest example of three-electron bonding can be found in the helium dimer
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak ...
cation, . It is considered a "half bond" because it consists of only one shared electron (rather than two); in molecular orbital terms, the third electron is in an anti-bonding orbital which cancels out half of the bond formed by the other two electrons. Another example of a molecule containing a 3-electron bond, in addition to two 2-electron bonds, is nitric oxide, NO. The oxygen molecule, O2 can also be regarded as having two 3-electron bonds and one 2-electron bond, which accounts for its paramagnetism
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
and its formal bond order of 2. Chlorine dioxide
Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually ...
and its heavier analogues bromine dioxide
Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO2. It forms unstable yellow to yellow-orange crystals. It was first isolated by R. Schwarz and M. Schmeißer in 1937 and is hypothesized to be important ...
and iodine dioxide also contain three-electron bonds.
Molecules with odd-electron bonds are usually highly reactive. These types of bond are only stable between atoms with similar electronegativities.
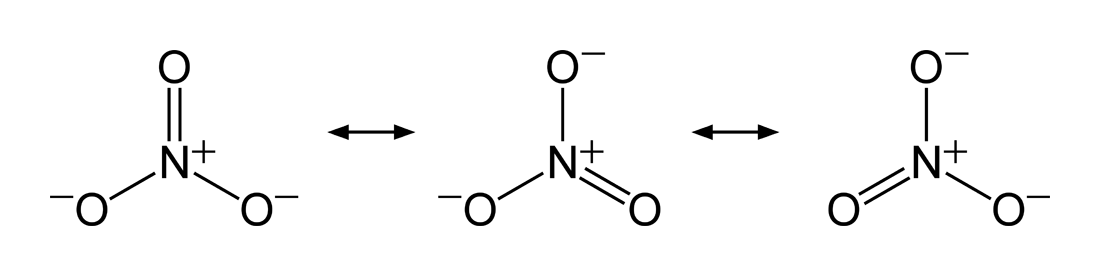
Resonance
There are situations whereby a singleLewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
is insufficient to explain the electron configuration in a molecule and its resulting experimentally-determined properties, hence a superposition of structures is needed. The same two atoms in such molecules can be bonded differently in different Lewis structures (a single bond in one, a double bond in another, or even none at all), resulting in a non-integer bond order. The nitrate ion is one such example with three equivalent structures. The bond between the nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and each oxygen is a double bond in one structure and a single bond in the other two, so that the average bond order for each N–O interaction is = .

Aromaticity
Inorganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, when a molecule with a planar ring obeys Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was ...
, where the number of π electrons fit the formula 4''n'' + 2 (where ''n'' is an integer), it attains extra stability and symmetry. In benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
, the prototypical aromatic compound, there are 6 π bonding electrons (''n'' = 1, 4''n'' + 2 = 6). These occupy three delocalized π molecular orbitals (molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecul ...
) or form conjugate π bonds in two resonance structures that linearly combine ( valence bond theory), creating a regular hexagon
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
exhibiting a greater stabilization than the hypothetical 1,3,5-cyclohexatriene.
In the case of heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
aromatics and substituted benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
s, the electronegativity differences between different parts of the ring may dominate the chemical behavior of aromatic ring bonds, which otherwise are equivalent.
Hypervalence
Certain molecules such asxenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherw ...
and sulfur hexafluoride have higher co-ordination numbers than would be possible due to strictly covalent bonding according to the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rul ...
. This is explained by the three-center four-electron bond
The 3-center 4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur tetrafluoride, the xenon fluorides, and the bifluoride ion. It is also know ...
("3c–4e") model which interprets the molecular wavefunction in terms of non-bonding highest occupied molecular orbital
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
s in molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecul ...
and resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied Periodic function, periodic force (or a Fourier analysis, Fourier component of it) is equal or close to a natural frequency of the system ...
of sigma bonds in valence bond theory.
Electron deficiency
Inthree-center two-electron bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''-b ...
s ("3c–2e") three atoms share two electrons in bonding. This type of bonding occurs in boron hydrides
Boranes is the name given to compounds with the formula BxHy and related anions. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bond ...
such as diborane
Diborane(6), generally known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracte ...
(B2H6), which are often described as electron deficient because there are not enough valence electrons to form localized (2-centre 2-electron) bonds joining all the atoms. However the more modern description using 3c–2e bonds does provide enough bonding orbitals to connect all the atoms, so that the molecules can instead be classified as electron-precise.
Each such bond (2 per molecule in diborane) contains a pair of electrons which connect the boron atoms to each other in a banana shape, with a proton (the nucleus of a hydrogen atom) in the middle of the bond, sharing electrons with both boron atoms. In certain cluster compounds, so-called four-center two-electron bond A 4-center 2-electron (4c–2e) bond is a type of chemical bond in which four atoms share two electrons in bonding, with a net bond order of . This type of bonding differs from the usual covalent bond, which involves two atoms sharing two electrons ...
s also have been postulated.
Quantum mechanical description
After the development of quantum mechanics, two basic theories were proposed to provide a quantum description of chemical bonding: valence bond (VB) theory and molecular orbital (MO) theory. A more recent quantum description is given in terms of atomic contributions to the electronic density of states.Comparison of VB and MO theories
The two theories represent two ways to build up the electron configuration of the molecule. For valence bond theory, the atomichybrid orbitals
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
are filled with electrons first to produce a fully bonded valence configuration, followed by performing a linear combination of contributing structures (resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied Periodic function, periodic force (or a Fourier analysis, Fourier component of it) is equal or close to a natural frequency of the system ...
) if there are several of them. In contrast, for molecular orbital theory a linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefun ...
is performed first, followed by filling of the resulting molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
s with electrons.
The two approaches are regarded as complementary, and each provides its own insights into the problem of chemical bonding. As valence bond theory builds the molecular wavefunction out of localized bonds, it is more suited for the calculation of bond energies and the understanding of reaction mechanisms. As molecular orbital theory builds the molecular wavefunction out of delocalized orbitals, it is more suited for the calculation of ionization energies
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
and the understanding of spectral absorption bands.
At the qualitative level, both theories contain incorrect predictions. Simple (Heitler–London) valence bond theory correctly predicts the dissociation of homonuclear diatomic molecules into separate atoms, while simple (Hartree–Fock) molecular orbital theory incorrectly predicts dissociation into a mixture of atoms and ions. On the other hand, simple molecular orbital theory correctly predicts Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was ...
of aromaticity, while simple valence bond theory incorrectly predicts that cyclobutadiene has larger resonance energy than benzene.
Although the wavefunctions generated by both theories at the qualitative level do not agree and do not match the stabilization energy by experiment, they can be corrected by configuration interaction
Configuration interaction (CI) is a post-Hartree–Fock linear variational method for solving the nonrelativistic Schrödinger equation within the Born–Oppenheimer approximation for a quantum chemical multi-electron system. Mathematical ...
. This is done by combining the valence bond covalent function with the functions describing all possible ionic structures or by combining the molecular orbital ground state function with the functions describing all possible excited states using unoccupied orbitals. It can then be seen that the simple molecular orbital approach overestimates the weight of the ionic structures while the simple valence bond approach neglects them. This can also be described as saying that the simple molecular orbital approach neglects electron correlation
Electronic correlation is the interaction between electrons in the electronic structure of a quantum system. The correlation energy is a measure of how much the movement of one electron is influenced by the presence of all other electrons.
Ato ...
while the simple valence bond approach overestimates it.
Modern calculations in quantum chemistry usually start from (but ultimately go far beyond) a molecular orbital rather than a valence bond approach, not because of any intrinsic superiority in the former but rather because the MO approach is more readily adapted to numerical computations. Molecular orbitals are orthogonal, which significantly increases the feasibility and speed of computer calculations compared to nonorthogonal valence bond orbitals.
Covalency from atomic contribution to the electronic density of states
In COOP, COHP and BCOOP, evaluation of bond covalency is dependent on the basis set. To overcome this issue, an alternative formulation of the bond covalency can be provided in this way. The center mass of an atomic orbital with quantum numbers for atom A is defined as : where is the contribution of the atomic orbital of the atom A to the total electronic density of states of the solid : where the outer sum runs over all atoms A of the unit cell. The energy window is chosen in such a way that it encompasses all of the relevant bands participating in the bond. If the range to select is unclear, it can be identified in practice by examining the molecular orbitals that describe the electron density along with the considered bond. The relative position of the center mass of levels of atom A with respect to the center mass of levels of atom B is given as : where the contributions of the magnetic and spin quantum numbers are summed. According to this definition, the relative position of the A levels with respect to the B levels is : where, for simplicity, we may omit the dependence from the principal quantum number in the notation referring to In this formalism, the greater the value of the higher the overlap of the selected atomic bands, and thus the electron density described by those orbitals gives a more covalent bond. The quantity is denoted as the ''covalency'' of the bond, which is specified in the same units of the energy .Analogous effect in nuclear systems
An analogous effect to covalent binding is believed to occur in some nuclear systems, with the difference that the shared fermions arequarks
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei. All commonly ...
rather than electrons. High energy proton-proton scattering cross-section indicates that quark interchange of either u or d quarks is the dominant process of the nuclear force
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nucle ...
at short distance. In particular, it dominates over the Yukawa interaction
In particle physics, Yukawa's interaction or Yukawa coupling, named after Hideki Yukawa, is an interaction between particles according to the Yukawa potential. Specifically, it is a scalar field (or pseudoscalar field) and a Dirac field of the ...
where a meson
In particle physics, a meson ( or ) is a type of hadronic subatomic particle composed of an equal number of quarks and antiquarks, usually one of each, bound together by the strong interaction. Because mesons are composed of quark subparticles, ...
is exchanged. Therefore, covalent binding by quark interchange is expected to be the dominating mechanism of nuclear binding at small distance when the bound hadrons
In particle physics, a hadron (; grc, ἁδρός, hadrós; "stout, thick") is a composite subatomic particle made of two or more quarks held together by the strong interaction. They are analogous to molecules that are held together by the ele ...
have covalence quarks in common.
See also
*Bonding in solids
Solids can be classified according to the nature of the bonding between their atomic or molecular components. The traditional classification distinguishes four kinds of bonding:
* Covalent bonding, which forms network covalent solids (sometimes ...
* Bond order
* Coordinate covalent bond, also known as a dipolar bond or a dative covalent bond
* Covalent bond classification (or LXZ notation)
* Covalent radius
The covalent radius, ''r''cov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm) or angstroms (Å), with 1 Å = 100 pm.
In principle, the sum of the two coval ...
* Disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
* Hybridization
* Hydrogen bond
* Ionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
* Linear combination of atomic orbitals
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefun ...
* Metallic bonding
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be des ...
* Noncovalent bonding
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. Th ...
* Resonance (chemistry)
References
Sources
* * *External links
Covalent Bonds and Molecular Structure
{{Authority control Chemical bonding