carboxylic acid reduction on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 For reductions of aldehydes and ketones, an aluminium hydride ion reduces the compound to form an alkoxide salt. After the complete reduction, the alkoxide is protonated to give the alcohol product:
For reductions of aldehydes and ketones, an aluminium hydride ion reduces the compound to form an alkoxide salt. After the complete reduction, the alkoxide is protonated to give the alcohol product:

 Ketones are less reactive than aldehydes, because of greater steric effects, and because the extra alkyl group can donate electron density to the partial positive charge of the polar C=O bond. Therefore, aldehydes reduce more easily than ketones and require milder reagents and milder conditions. Carboxylic acids and esters are further stabilized by the presence of a second oxygen atom which can donate a lone pair into the already polar C=O bond. Acyl halides are the least stable of the carbonyls since halides are poor
Ketones are less reactive than aldehydes, because of greater steric effects, and because the extra alkyl group can donate electron density to the partial positive charge of the polar C=O bond. Therefore, aldehydes reduce more easily than ketones and require milder reagents and milder conditions. Carboxylic acids and esters are further stabilized by the presence of a second oxygen atom which can donate a lone pair into the already polar C=O bond. Acyl halides are the least stable of the carbonyls since halides are poor
 The relatively weak reducer sodium borohydride is typically used for reducing ketones and aldehydes because unlike lithium aluminum hydride, it tolerates many functional groups (nitro group, nitrile, ester) and can be used with water or ethanol as solvents. Lithium aluminum hydride and other strong reducers such as diisobutylaluminium hydride, L-selectride, diborane, diazene, and aluminum hydride can also reduce aldehydes and ketones, but are disfavored because they are hazardous and violently reactive. However, these compounds are useful for reducing carboxylic acids and esters to alcohols, since sodium borohydride is not powerful enough to do so.
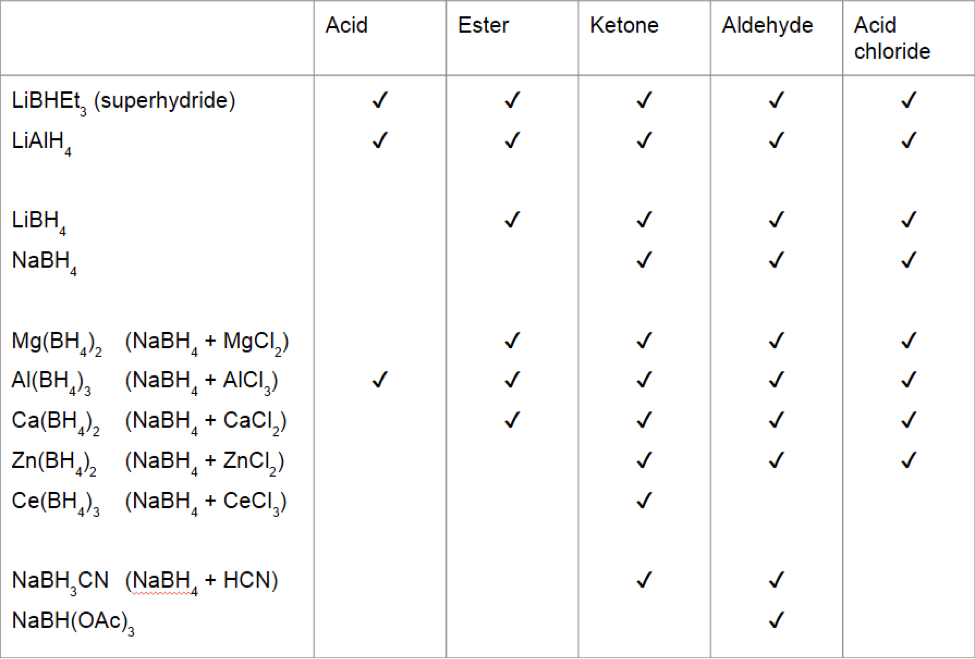
The following table illustrates which carbonyl functional groups can be reduced by which reducing agents (some of these reagents vary in efficacy depending on reaction conditions):
The relatively weak reducer sodium borohydride is typically used for reducing ketones and aldehydes because unlike lithium aluminum hydride, it tolerates many functional groups (nitro group, nitrile, ester) and can be used with water or ethanol as solvents. Lithium aluminum hydride and other strong reducers such as diisobutylaluminium hydride, L-selectride, diborane, diazene, and aluminum hydride can also reduce aldehydes and ketones, but are disfavored because they are hazardous and violently reactive. However, these compounds are useful for reducing carboxylic acids and esters to alcohols, since sodium borohydride is not powerful enough to do so.
The following table illustrates which carbonyl functional groups can be reduced by which reducing agents (some of these reagents vary in efficacy depending on reaction conditions):
 In the
In the  The Weinreb amide is reduced via a stable
The Weinreb amide is reduced via a stable  The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
 Aldehydes and ketones can be reduced not only to alcohols but also to alkanes. Some reactions for this transformation include the
Aldehydes and ketones can be reduced not only to alcohols but also to alkanes. Some reactions for this transformation include the
 In α,β-reduction (also called conjugate reduction), the substrate is an α,β-unsaturated carbonyl, an enone or enal.
When these substrates are reduced, 1,2-reduction - which produces an
In α,β-reduction (also called conjugate reduction), the substrate is an α,β-unsaturated carbonyl, an enone or enal.
When these substrates are reduced, 1,2-reduction - which produces an  The more sterically hindered the enone substrate, the more likely 1,2 reduction becomes. Additionally, to selectively form the alcohol and avoid the 1,4 product, the
The more sterically hindered the enone substrate, the more likely 1,2 reduction becomes. Additionally, to selectively form the alcohol and avoid the 1,4 product, the 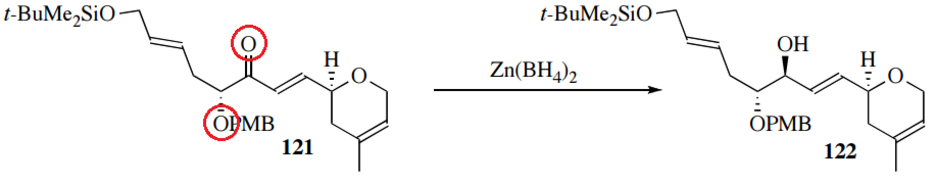
 Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, carbonyl reduction is the organic reduction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carr ...
of any carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
group by a reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth met ...
.
Typical carbonyl compounds are ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double b ...
s, aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl gro ...
s, carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
s, ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s, and acid halides. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohol
A primary alcohol is an alcohol in which the hydroxy group is bonded to a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH” group.
In contrast, a secondary alcohol has a formula “–CHROH” and a tertiary a ...
s, depending on the strength of the reducing agent; aldehydes and ketones can be reduced respectively to primary and secondary alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
. In deoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to orga ...
, the alcohol can be further reduced and removed altogether.
Metal hydrides
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
based on boron and aluminum are common reducing agents; catalytic hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
is also an important method of reducing carbonyls. Before the discovery of soluble hydride reagents, esters were reduced by the Bouveault–Blanc reduction
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc de ...
, employing a mixture of sodium metal in the presence of alcohols.
Carboxylic acid derivatives, aldehydes, and ketones to alcohols
Hydride reduction
Mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
for metal hydride reduction is based on nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions d ...
of hydride to the carbonyl carbon. In some cases, the alkali metal cation, especially Li+, activates the carbonyl group by coordinating to the carbonyl oxygen, thereby enhancing the electrophilicity of the carbonyl.
For reductions of carboxylic acid derivatives, after reduction by an aluminium hydride ion, an elimination leads to the aldehyde product (which can be reduced a second time to an alcohol):
 For reductions of aldehydes and ketones, an aluminium hydride ion reduces the compound to form an alkoxide salt. After the complete reduction, the alkoxide is protonated to give the alcohol product:
For reductions of aldehydes and ketones, an aluminium hydride ion reduces the compound to form an alkoxide salt. After the complete reduction, the alkoxide is protonated to give the alcohol product:

Trends in carbonyl reactivity
 Ketones are less reactive than aldehydes, because of greater steric effects, and because the extra alkyl group can donate electron density to the partial positive charge of the polar C=O bond. Therefore, aldehydes reduce more easily than ketones and require milder reagents and milder conditions. Carboxylic acids and esters are further stabilized by the presence of a second oxygen atom which can donate a lone pair into the already polar C=O bond. Acyl halides are the least stable of the carbonyls since halides are poor
Ketones are less reactive than aldehydes, because of greater steric effects, and because the extra alkyl group can donate electron density to the partial positive charge of the polar C=O bond. Therefore, aldehydes reduce more easily than ketones and require milder reagents and milder conditions. Carboxylic acids and esters are further stabilized by the presence of a second oxygen atom which can donate a lone pair into the already polar C=O bond. Acyl halides are the least stable of the carbonyls since halides are poor electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent chemi ...
s, as well as great leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
s.
The result of these trends in carbonyl reactivity is that acid halides, ketones, and aldehydes are usually the most readily reduced compounds, while acids and esters require stronger reducing.
Trends in metal hydride reactivity
Four major factors contribute to the strength of metal hydride reducing agents. First, the counter ion’s ability to activate carbonyls depends on how well it can coordinate to the carbonyl oxygen. Lithium is smaller and more electrophilic than sodium, so it coordinates much more strongly and activates the carbonyl more. Metals that can have multiple charges (such as Mg, Al, and Zn) form cations with high charge density, and are therefore also stronger activators than Na+. Second, the central metal can influence a reducing agent’s strength. Aluminum is larger than boron, so it bonds more weakly to hydrides, which are more free to attack; aluminum hydrides are therefore better reducers than borohydrides. A third factor, sterics, is what makes certain substituted hydrides (hydrides in which one or more hydrides are replaced by substituents) much weaker reducers than other metal hydrides:sodium triacetoxyborohydride
Sodium triacetoxyborohydride, also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a chemical compound with the formula Na(CH3COO)3BH. Like other borohydrides, it is used as a reducing agent in organic synthesis. This colourle ...
(NaBH(OAc)3), for instance, can be used to selectively reduce aldehydes, and leave the less reactive ketones unreacted.
Finally, substituents can have other effects on a reducing agent’s reactivity: acetoxy groups hinder the reducing power of NaBH(OAc)3 not only through steric bulk but also because they are electron-withdrawing. Cyano groups also hinder reducing agents, while electron-donating groups such as alkyl groups can improve them, such as in superhydride (lithium triethylborohydride), which is a strong enough nucleophile to prevent undesired rearrangements during reduction.
Because of these substituent effects, NaBH3CN is a very poor reducer at moderate pH (>4), so it prefers reductive amination to carbonyl reduction, as shown below:
 The relatively weak reducer sodium borohydride is typically used for reducing ketones and aldehydes because unlike lithium aluminum hydride, it tolerates many functional groups (nitro group, nitrile, ester) and can be used with water or ethanol as solvents. Lithium aluminum hydride and other strong reducers such as diisobutylaluminium hydride, L-selectride, diborane, diazene, and aluminum hydride can also reduce aldehydes and ketones, but are disfavored because they are hazardous and violently reactive. However, these compounds are useful for reducing carboxylic acids and esters to alcohols, since sodium borohydride is not powerful enough to do so.
The following table illustrates which carbonyl functional groups can be reduced by which reducing agents (some of these reagents vary in efficacy depending on reaction conditions):
The relatively weak reducer sodium borohydride is typically used for reducing ketones and aldehydes because unlike lithium aluminum hydride, it tolerates many functional groups (nitro group, nitrile, ester) and can be used with water or ethanol as solvents. Lithium aluminum hydride and other strong reducers such as diisobutylaluminium hydride, L-selectride, diborane, diazene, and aluminum hydride can also reduce aldehydes and ketones, but are disfavored because they are hazardous and violently reactive. However, these compounds are useful for reducing carboxylic acids and esters to alcohols, since sodium borohydride is not powerful enough to do so.
The following table illustrates which carbonyl functional groups can be reduced by which reducing agents (some of these reagents vary in efficacy depending on reaction conditions):

Carboxylic acid derivatives to aldehydes
Using metal hydrides
Forming aldehydes from carboxylic acid derivatives is often a challenge, because weaker reducing agents (NaBH4) are incapable of reducing esters and carboxylic acids, which are relatively stable, and stronger reducing agents (LiAlH4) immediately reduce the formed aldehyde to an alcohol. Since acid chlorides are less stable than aldehydes and ketones, they are often used in conjunction with sterically hindered anhydride donors when synthesizing aldehydes, because the relatively weak reducer will react preferentially with the acid chloride starting material, leaving the aldehyde product unreacted. The reducing agentDIBAL-H
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (''i''-Bu2AlH)2, where ''i''-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis.
Properties
Lik ...
(Diisobutylaluminium hydride) is often used for this purpose: though it normally reduces all carbonyls, it can stop reducing at the aldehyde if only one equivalent is used at low temperatures. LiAl(OtBu)3 (formed from LiAlH4 and tBuOH in situ) can also stop reducing at the aldehyde, through a similar mechanism to DIBAL-H.
Alternative methods
The traditional method of forming aldehydes without reducing to alcohols - by using hindered hydrides and reactive carbonyls - is limited by its narrow substrate scope and great dependence on reaction conditions. One workaround to avoid this method is to reduce the carboxylic acid derivative all the way down to an alcohol, then oxidize the alcohol back to an aldehyde. Other alternatives include forming a thioester or a Weinreb amide, then reducing the new species to an aldehyde through the Fukuyama reduction or Weinreb reaction respectively, or using catalytic hydrogenation as in the Rosenmund reaction. In the
In the Fukuyama reduction
The Fukuyama reduction is an organic reaction and an organic reduction in which a thioester is reduced to an aldehyde by a silyl hydride in presence of a catalytic amount of palladium. This reaction was invented in 1990 by Tohru Fukuyama. In t ...
, a carboxylic acid is first converted to a thioester through addition of a thiol (with a mechanism similar to esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
). The thioester is then reduced to an aldehyde by a silyl hydride with a palladium catalyst.
In the Weinreb ketone synthesis
The Weinreb–Nahm ketone synthesis is a chemical reaction used in organic chemistry to make carbon–carbon bonds. It was discovered in 1981 by Steven M. Weinreb and Steven Nahm as a method to synthesize ketones. The original reaction involved ...
, an acyl chloride is first converted to the Weinreb amide, then treated with an organometallic reagent to form a ketone, or lithium aluminum hydride to form an aldehyde:
 The Weinreb amide is reduced via a stable
The Weinreb amide is reduced via a stable chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
, rather than the electrophilic carbonyl that is formed through metal hydride reductions; the chelate is therefore only reduced once, as illustrated below:
 The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
The Rosenmund reaction reduces acyl chlorides to aldehydes using hydrogen gas with a catalyst of palladium on barium sulfate, whose small surface area prevents over-reduction.
Aldehydes and ketones to alkanes
 Aldehydes and ketones can be reduced not only to alcohols but also to alkanes. Some reactions for this transformation include the
Aldehydes and ketones can be reduced not only to alcohols but also to alkanes. Some reactions for this transformation include the Clemmensen reduction
Clemmensen reduction is a chemical reaction described as a reduction of ketones (or aldehydes) to alkanes using zinc amalgam and concentrated hydrochloric acid. This reaction is named after Erik Christian Clemmensen, a Danish chemist.
The orig ...
(in strongly acidic conditions) and the Wolff–Kishner reduction
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has se ...
(in strongly basic conditions), as well as the various modifications of Wolff-Kishner reaction. The Caglioti modification, for instance, uses tosylhydrazone with a hydride donor in milder conditions with no base; the Myers modification substitutes hydrazine with bis(tert-butyldimethylsilyl)-hydrazine, uses milder conditions at room temperature, and is rapid and efficient.
Aromatic carbonyls
Aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
carbonyls are more readily reduced to their respective alkanes than aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane ...
compounds. For example, ketones are reduced to their respective alkyl benzenes by catalytic hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
or by Birch reduction
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally ...
under mild conditions.
α,β-unsaturated carbonyls
allyl alcohol
Allyl alcohol ( IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula . Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a raw material ...
- is in competition with the 1,4-reduction - which forms the saturated ketone or aldehyde. The following NaBH4 reduction of an enone shows two possible products: the first from 1,4-reduction and the second from 1,2-reduction. The more sterically hindered the enone substrate, the more likely 1,2 reduction becomes. Additionally, to selectively form the alcohol and avoid the 1,4 product, the
The more sterically hindered the enone substrate, the more likely 1,2 reduction becomes. Additionally, to selectively form the alcohol and avoid the 1,4 product, the Luche reduction
Luche reduction is the selective organic reduction of α,β-unsaturated ketones to allylic alcohols with sodium borohydride (NaBH4) and lanthanide chlorides, mainly cerium(III) chloride (CeCl3), in methanol or ethanol. The Luche reduction can ...
uses the bigger molecule Ce(BH4)3 (derived from NaBH4 and CeCl3 combined in situ) as the hydride source.
The hydride source Zn(BH4)2 also shows 1,2 selectivity, as well as greater diastereoselectivity; it does so by coordinating not only to the carbonyl oxygen but also to adjacent atoms:

Stereoselectivity
Diastereoselective reduction
In the reduction of cyclohexanones, the hydride source can attack axially to produce an equatorial alcohol, or equatorially to produce an axial alcohol. In axial attack (shown in red), the hydride encounters 1,3-diaxial strain. In equatorial attack (shown in blue), the hydride avoids the 1,3-diaxial interaction, but the substrate undergoes unfavorable torsional strain when the newly formed alcohol and added hydrogen atom eclipse each other in the reaction intermediate (as shown in the Newman projection for the axial alcohol). Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
Large reducing agents, such as LiBH(Me2CHCHMe)3, are hindered by the 1,3-axial interactions and therefore attack equatorially. Small reducing agents, such as NaBH4, preferentially attack axially in order to avoid the eclipsing interactions, because the 1,3-diaxial interaction for small molecules is minimal; stereoelectronic reasons have also been cited for small reducing agents' axial preference. Making the substrate bulkier (and increasing 1,3-axial interactions), however, decreases the prevalence of axial attacks, even for small hydride donors.
Enantioselective reduction
When asymmetrical ketones are reduced, the resulting secondary alcohol has a chiral center whose can be controlled using chiral catalysts. Well-known carbonyl reductions inasymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
are the Noyori asymmetric hydrogenation
In chemistry, the Noyori asymmetric hydrogenation refers to methodology for enantioselective reduction of ketones and related functional groups. This methodology was introduced by Ryoji Noyori, who shared the Nobel Prize in Chemistry in 2001 for c ...
(beta-ketoester reduction/Ru/BINAP) and the CBS reduction
CBS Broadcasting Inc., commonly shortened to CBS, the abbreviation of its former legal name Columbia Broadcasting System, is an American commercial broadcast television and radio network serving as the flagship property of the CBS Entertainme ...
(BH3, proline derived chiral catalyst).
See also
*Baker's yeast
Baker's yeast is the common name for the strains of yeast commonly used in baking bread and other bakery products, serving as a leavening agent which causes the bread to rise (expand and become lighter and softer) by converting the fermentabl ...
, a biotransformation Biotransformation is the biochemical modification of one chemical compound or a mixture of chemical compounds. Biotransformations can be conducted with whole cells, their lysates, or purified enzymes. Increasingly, biotransformations are effected w ...
route for carbonyl reductions.
References
{{Organic reactions Organic redox reactions Nucleophilic addition reactions