Butadiene on:
[Wikipedia]
[Google]
[Amazon]
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest
 This process was the basis for the Soviet Union's synthetic rubber industry during and after World War II, and it remained in limited use in Russia and other parts of eastern Europe until the end of the 1970s. At the same time this type of manufacture was canceled in Brazil. As of 2017, no butadiene was produced industrially from ethanol.
In the other, two-step process, developed by the Russian emigre chemist
This process was the basis for the Soviet Union's synthetic rubber industry during and after World War II, and it remained in limited use in Russia and other parts of eastern Europe until the end of the 1970s. At the same time this type of manufacture was canceled in Brazil. As of 2017, no butadiene was produced industrially from ethanol.
In the other, two-step process, developed by the Russian emigre chemist  This process was one of the three used in the United States to produce "government rubber" during World War II, although it is less economical than the butane or butene routes for the large volumes. Still, three plants with a total capacity of 200,000 tons per year were constructed in the U.S. (
This process was one of the three used in the United States to produce "government rubber" during World War II, although it is less economical than the butane or butene routes for the large volumes. Still, three plants with a total capacity of 200,000 tons per year were constructed in the U.S. (
 The most stable
The most stable 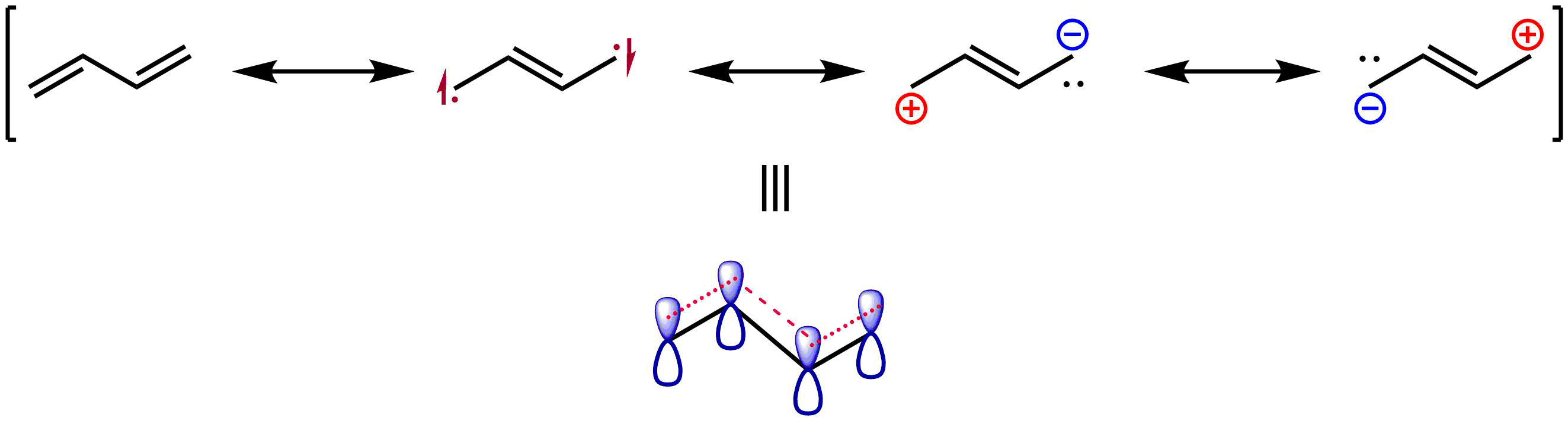 A qualitative picture of the molecular orbitals of 1,3-butadiene is readily obtained by applying Hückel theory. (The article on Hückel theory gives a derivation for the butadiene orbitals.)
1,3-Butadiene is also thermodynamically stabilized. While a monosubstituted double bond releases about 30.3 kcal/mol of heat upon hydrogenation, 1,3-butadiene releases slightly less (57.1 kcal/mol) than twice this energy (60.6 kcal/mol), expected for two isolated double bonds. That implies a stabilization energy of 3.5 kcal/mol. Similarly, the hydrogenation of the terminal double bond of 1,4-pentadiene releases 30.1 kcal/mol of heat, while hydrogenation of the terminal double bond of conjugated (''E'')-1,3-pentadiene releases only 26.5 kcal/mol, implying a very similar value of 3.6 kcal/mol for the stabilization energy. The ~3.5 kcal/mol difference in these heats of hydrogenation can be taken to be the resonance energy of a conjugated diene.
A qualitative picture of the molecular orbitals of 1,3-butadiene is readily obtained by applying Hückel theory. (The article on Hückel theory gives a derivation for the butadiene orbitals.)
1,3-Butadiene is also thermodynamically stabilized. While a monosubstituted double bond releases about 30.3 kcal/mol of heat upon hydrogenation, 1,3-butadiene releases slightly less (57.1 kcal/mol) than twice this energy (60.6 kcal/mol), expected for two isolated double bonds. That implies a stabilization energy of 3.5 kcal/mol. Similarly, the hydrogenation of the terminal double bond of 1,4-pentadiene releases 30.1 kcal/mol of heat, while hydrogenation of the terminal double bond of conjugated (''E'')-1,3-pentadiene releases only 26.5 kcal/mol, implying a very similar value of 3.6 kcal/mol for the stabilization energy. The ~3.5 kcal/mol difference in these heats of hydrogenation can be taken to be the resonance energy of a conjugated diene.
EPA website
/ref> 1,3-Butadiene is recognized as a Highly Reactive Volatile Organic Compound (HRVOC) for its potential to readily form ozone, and as such, emissions of the chemical are highly regulated by
1,3-Butadiene
– Agency for Toxic Substances and Disease Registry
– CDC - NIOSH Pocket Guide to Chemical Hazards
{{DEFAULTSORT:Butadiene, 1, 3- Alkadienes Hazardous air pollutants Monomers IARC Group 1 carcinogens U.S. Synthetic Rubber Program Commodity chemicals Petrochemicals Conjugated dienes
conjugated diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclatur ...
.
Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles.
The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which ...
has no industrial significance.
History
In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis ofamyl alcohol
An amyl alcohol is any of eight alcohols with the formula C5H12O. A mixture of amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol. Amyl alcohol is used as a solvent and in esterification, by which is produced amyl acetat ...
. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong
Henry Edward Armstrong FRS FRSE (Hon) (6 May 1848 – 13 July 1937) was a British chemist. Although Armstrong was active in many areas of scientific research, such as the chemistry of naphthalene derivatives, he is remembered today largely for h ...
isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a material with rubber-like properties. This polymer was, however, found to be too soft to replace natural rubber in many applications, notably automobile tires.
The butadiene industry originated in the years leading up to World War II. Many of the belligerent nations realized that in the event of war, they could be cut off from rubber plantations controlled by the British Empire
The British Empire was composed of the dominions, colonies, protectorates, mandates, and other territories ruled or administered by the United Kingdom and its predecessor states. It began with the overseas possessions and trading posts esta ...
, and sought to reduce their dependence on natural rubber. In 1929, Eduard Tschunker
Eduard Model Accessories is a Czech manufacturer of plastic models and finescale model accessories.
Formed in 1989 in the city of Most, Eduard began in a rented cellar as a manufacturer of photoetched brass model components. Following the succ ...
and Walter Bock
Walter Bock (20 January 1895 – 25 October 1948)Death record Nr. 3271/Köln I for Ludwig Walter Robert Bock of Oct. 26, 1948, Landesarchiv NRW, Duisburg was a German chemist who developed styrene-butadiene copolymer by emulsion polymerization ...
, working for IG Farben in Germany, made a copolymer of styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
and butadiene that could be used in automobile tires. Worldwide production quickly ensued, with butadiene being produced from grain alcohol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hyd ...
in the Soviet Union and the United States, and from coal-derived acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure f ...
in Germany.
Production
Extraction from C4 hydrocarbons
In the United States, western Europe, and Japan, butadiene is produced as a byproduct of the steam cracking process used to produceethylene
Ethylene ( IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
and other alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
s. When mixed with steam and briefly heated to very high temperatures (often over 900 °C), aliphatic hydrocarbons give up hydrogen to produce a complex mixture of unsaturated hydrocarbons, including butadiene. The quantity of butadiene produced depends on the hydrocarbons used as feed. Light feeds, such as ethane, give primarily ethylene
Ethylene ( IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
when cracked, but heavier feeds favor the formation of heavier olefins, butadiene, and aromatic hydrocarbon
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
s.
Butadiene is typically isolated from the other four-carbon hydrocarbons produced in steam cracking by extractive distillation using a polar aprotic solvent A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these solvents do not serve as proton donors in hydrogen bonding
In chemistry, a hydro ...
such as acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed ...
, ''N''-methyl-2-pyrrolidone, furfural, or dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the major ...
, from which it is then stripped by distillation.Sun, H.P. Wristers, J.P. (1992). Butadiene. In J.I. Kroschwitz (Ed.), ''Encyclopedia of Chemical Technology, 4th ed.'', vol. 4, pp. 663–690. New York: John Wiley & Sons.
From dehydrogenation of ''n''-butane
Butadiene can also be produced by the catalytic dehydrogenation of normal butane (''n''-butane). The first such post-war commercial plant, producing 65,000ton
Ton is the name of any one of several units of measure. It has a long history and has acquired several meanings and uses.
Mainly it describes units of weight. Confusion can arise because ''ton'' can mean
* the long ton, which is 2,240 pounds
...
s per year of butadiene, began operations in 1957 in Houston, Texas.Beychok, M.R. and Brack, W.J., "First Postwar Butadiene Plant", ''Petroleum Refiner'', June 1957. Prior to that, in the 1940s the Rubber Reserve Company, a part of the United States government, constructed several plants in Borger, Texas
Borger ( ) is the largest city in Hutchinson County, Texas, United States. The population was 12,551 at the 2020 census. Borger is named for businessman Asa Philip "Ace" Borger, who also established the Hutchinson County seat of Stinnett ...
, Toledo, Ohio, and El Segundo, California, to produce synthetic rubber for the war effort as part of the United States Synthetic Rubber Program.Herbert, Vernon, "Synthetic Rubber: A Project That Had to Succeed", Greenwood Press, 1985, Total capacity was 68 KMTA (Kilo Metric Tons per Annum).
Today, butadiene from ''n''-butane is commercially produced using the Houdry Catadiene process, which was developed during World War II. This entails treating butane over alumina and chromia
In Greek mythology, Chromia (; Ancient Greek: , ''Khrōmía'') was the daughter of Itonus, son of Amphictyon, himself son of Deucalion. She was also, in some traditions, the mother of Aetolus, Paeon, Epeius and Eurycyda by Endymion.
The poe ...
at high temperatures.
From ethanol
In other parts of the world, including South America, Eastern Europe, China, and India, butadiene is also produced from ethanol. While not competitive with steam cracking for producing large volumes of butadiene, lower capital costs make production from ethanol a viable option for smaller-capacity plants. Two processes were in use. In the single-step process developed by Sergei Lebedev, ethanol is converted to butadiene, hydrogen, and water at 400–450 °C over any of a variety of metal oxide catalysts:Kirshenbaum, I. (1978). Butadiene. In M. Grayson (Ed.), ''Encyclopedia of Chemical Technology, 3rd ed.'', vol. 4, pp. 313–337. New York: John Wiley & Sons. :Ivan Ostromislensky
Ivan Ivanovich Ostromislensky (russian: Иван Иванович Остромысленский, also Iwan Ostromislensky) (9 September 1880 – 16 January 1939) was a Russian organic chemist. He is credited as the pioneer in studying polymeriza ...
, ethanol is oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
to acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the m ...
, which reacts with additional ethanol over a tantalum-promoted porous silica catalyst at 325–350 °C to yield butadiene:
: This process was one of the three used in the United States to produce "government rubber" during World War II, although it is less economical than the butane or butene routes for the large volumes. Still, three plants with a total capacity of 200,000 tons per year were constructed in the U.S. (
This process was one of the three used in the United States to produce "government rubber" during World War II, although it is less economical than the butane or butene routes for the large volumes. Still, three plants with a total capacity of 200,000 tons per year were constructed in the U.S. (Institute, West Virginia
Institute is an unincorporated community on the Kanawha River in Kanawha County, West Virginia, United States. Interstate 64 and West Virginia Route 25 pass by the community, which has grown to intermingle with nearby Dunbar. As of 2018, the commu ...
, Louisville, Kentucky, and Kobuta, Pennsylvania Kobuta is an unincorporated community in Potter Township, Beaver County, Pennsylvania, United States. It is located along the Ohio River, due west of Monaca, southwest of Industry, and southwest of Beaver. The area was the site of a butadiene, ...
) with start-ups completed in 1943, the Louisville plant initially created butadiene from acetylene generated by an associated calcium carbide plant. The process remains in use today in China and India.
From butenes
1,3-Butadiene can also be produced by catalytic dehydrogenation of normalbutene
Butene, also known as butylene, is an alkene with the formula . The word ''butene'' may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for ...
s. This method was also used by the U.S. Synthetic Rubber Program
Kenneth Stanley "Boots" Adams (August 31, 1899 – March 30, 1975) was an American business executive, University of Kansas booster, and civic philanthropist of Bartlesville, Oklahoma. Adams began his career with the Phillips Petroleum Company in ...
(USSRP) during World War II. The process was much more economical than the alcohol or n-butane route but competed with aviation gasoline for available butene molecules (butenes were plentiful thanks to catalytic cracking
Fluid Catalytic Cracking (FCC) is the conversion process used in petroleum refineries to convert the high-boiling point, high-molecular weight hydrocarbon fractions of petroleum (crude oils) into gasoline, olefinic gases, and other petroleum pro ...
). The USSRP constructed several plants in Baton Rouge
Baton Rouge ( ; ) is a city in and the capital of the U.S. state of Louisiana. Located the eastern bank of the Mississippi River, it is the parish seat of East Baton Rouge Parish, Louisiana's most populous parish—the equivalent of counties ...
and Lake Charles, Louisiana; Houston, Baytown, and Port Neches, Texas
Port Neches is a city in Jefferson County, Texas, United States. The population was 13,692 at the 2020 census, up from 13,040 at the 2010 census. It is part of the Beaumont–Port Arthur metropolitan area.
History
The area known as Port Nech ...
; and Torrance, California
Torrance is a city in the Los Angeles metropolitan area located in Los Angeles County, California, United States. The city is part of what is known as the South Bay region of the metropolitan area. Torrance has of beachfront on the Pacific O ...
. Total annual production was 275 KMTA.
In the 1960s, a Houston company known as "Petro-Tex" patented a process to produce butadiene from normal butene
Butene, also known as butylene, is an alkene with the formula . The word ''butene'' may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for ...
s by oxidative dehydrogenation using a proprietary catalyst. It is unclear if this technology is practiced commercially.
After World War II, the production from butenes became the major type of production in USSR.
For laboratory use
1,3-Butadiene is inconvenient for laboratory use because it is gas. Laboratory procedures have been optimized for its generation from nongaseous precursors. It can be produced by the retro- Diels-Alder reaction of cyclohexene. Sulfolene is a convenient solid storable source for 1,3-butadiene in the laboratory. It releases the diene and sulfur dioxide upon heating.Uses
Most butadiene is used to make synthetic rubbers for the manufacture of tyres, grommets and elastic bands. The conversion of butadiene to synthetic rubbers is called polymerization, a process by which small molecules (monomers) are linked to make large ones (polymers). The mere polymerization of butadiene givespolybutadiene
Polybutadiene utadiene rubber BRis a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tir ...
, which is a very soft, almost liquid material. The polymerization of butadiene ''and'' other monomers gives copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
s, which are more valued. The polymerization of butadiene and styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
and/or acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular st ...
, such as acrylonitrile butadiene styrene
Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)''x''·(C4H6)''y''·(C3H3N)''z'' is a common thermoplastic polymer. Its glass transition temperature is approximately . ABS is amorphous and therefore has no true melting point.
AB ...
(ABS), nitrile-butadiene (NBR), and styrene-butadiene (SBR). These copolymers are tough and/or elastic depending on the ratio of the monomers used in their preparation. SBR is the material most commonly used for the production of automobile tyres. Precursors to still other synthetic rubbers are prepared from butadiene. One is chloroprene
Chloroprene is the common name for 2-chlorobuta-1,3-diene (IUPAC name) with the chemical formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, ...
.
Smaller amounts of butadiene are used to make adiponitrile
Adiponitrile is an organic compound with the chemical formula (CH2)4(CN)2. This viscous, colourless dinitrile is an important precursor to the polymer nylon 66. In 2005, about one million tonnes of adiponitrile were produced.M. T. Musser, "Adipi ...
, a precursor to some nylons. The conversion of butadiene to adiponitrile entails the addition of hydrogen cyanide to each of the double bonds in butadiene. The process is called hydrocyanation
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of pr ...
.
Butadiene is used to make the solvent sulfolane
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solvent ...
.
Butadiene is also useful in the synthesis of cycloalkanes
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure contain ...
and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through Diels-Alder reactions. The most widely used such reactions involve reactions of butadiene with one or two other molecules of butadiene, i.e., dimerization and trimerization respectively. Via dimerizatiion butadiene is converted to 4-vinylcyclohexene and cyclooctadiene
A cyclooctadiene (sometimes abbreviated COD) is any of several cyclic diene with the formula (CH2)4(C2H2)2. Focusing only on cis derivatives, four isomers are possible: 1,2-, which is an allene, 1,3-, 1,4-, and 1,5-. Commonly encountered isomers ar ...
. In fact, vinylcyclohexene is a common impurity that accumulates when butadiene is stored. Via trimerization, butadiene is converted to cyclododecatriene
Cyclododecatrienes are cyclic trienes with the formula C12H18. Four isomers are known for 1,5,9-cyclododecatriene. The ''trans'',''trans'',''cis''-isomer is a precursor in the production of nylon-12.
:
Production
The ''trans'',''trans'',''cis'' ...
. Some of these processes employ nickel- or titanium-containing catalysts.
Structure, conformation, and stability
 The most stable
The most stable conformer
Conformer is a clear acrylic shell fitted after an enucleation if the final artificial eye is not available at the time of surgery to hold the shape of the eye socket and allow the eyelids to blink over the shell without rubbing the suture line ...
of 1,3-butadiene is the ''s''-''trans'' conformation, in which the molecule is planar, with the two pairs of double bonds facing opposite directions. This conformation is most stable because orbital overlap between double bonds is maximized, allowing for maximum conjugation, while steric effects are minimized. Conventionally, the ''s-trans'' conformation is considered to have a C2-C3 dihedral angle of 180°. In contrast, the ''s''-''cis'' conformation, in which the dihedral angle is 0°, with the pair of double bonds facing the same direction is approximately 16.5 kJ/mol (3.9 kcal/mol) higher in energy, due to steric hindrance. This geometry is a local energy maximum, so in contrast to the ''s-trans'' geometry, it is not a conformer. The ''gauche'' geometry, in which the double bonds of the ''s-cis'' geometry are twisted to give a dihedral angle of around 38°, is a second conformer that is around 12.0 kJ/mol (2.9 kcal/mol) higher in energy than the ''s-trans'' conformer. Overall, there is a barrier of 24.8 kJ/mol (5.9 kcal/mol) for isomerization between the two conformers. This increased rotational barrier and strong overall preference for a near-planar geometry is evidence for a delocalized π system and a small degree of partial double bond character in the C–C single bond, in accord with resonance theory.
Despite the high energy of the ''s-cis'' conformation, 1,3-butadiene needs to assume this conformation (or one very similar) before it can participate as the four-electron component in concerted cycloaddition reactions like the Diels-Alder reaction.
Similarly, a combined experimental and computational study has found that the double bond of ''s-trans-''butadiene has a length of 133.8 pm, while that for ethylene has a length of 133.0 pm. This was taken as evidence of a π-bond weakened and lengthened by delocalization, as depicted by the resonance structures shown below.
 A qualitative picture of the molecular orbitals of 1,3-butadiene is readily obtained by applying Hückel theory. (The article on Hückel theory gives a derivation for the butadiene orbitals.)
1,3-Butadiene is also thermodynamically stabilized. While a monosubstituted double bond releases about 30.3 kcal/mol of heat upon hydrogenation, 1,3-butadiene releases slightly less (57.1 kcal/mol) than twice this energy (60.6 kcal/mol), expected for two isolated double bonds. That implies a stabilization energy of 3.5 kcal/mol. Similarly, the hydrogenation of the terminal double bond of 1,4-pentadiene releases 30.1 kcal/mol of heat, while hydrogenation of the terminal double bond of conjugated (''E'')-1,3-pentadiene releases only 26.5 kcal/mol, implying a very similar value of 3.6 kcal/mol for the stabilization energy. The ~3.5 kcal/mol difference in these heats of hydrogenation can be taken to be the resonance energy of a conjugated diene.
A qualitative picture of the molecular orbitals of 1,3-butadiene is readily obtained by applying Hückel theory. (The article on Hückel theory gives a derivation for the butadiene orbitals.)
1,3-Butadiene is also thermodynamically stabilized. While a monosubstituted double bond releases about 30.3 kcal/mol of heat upon hydrogenation, 1,3-butadiene releases slightly less (57.1 kcal/mol) than twice this energy (60.6 kcal/mol), expected for two isolated double bonds. That implies a stabilization energy of 3.5 kcal/mol. Similarly, the hydrogenation of the terminal double bond of 1,4-pentadiene releases 30.1 kcal/mol of heat, while hydrogenation of the terminal double bond of conjugated (''E'')-1,3-pentadiene releases only 26.5 kcal/mol, implying a very similar value of 3.6 kcal/mol for the stabilization energy. The ~3.5 kcal/mol difference in these heats of hydrogenation can be taken to be the resonance energy of a conjugated diene.
Reactions
The industrial uses illustrate the tendency of butadiene to polymerize. Its susceptibility to 1,4-addition reactions is illustrated by its hydrocyanation. Like many dienes, it undergoes Pd-catalyzed reactions that proceed via allyl complexes. It is a partner in Diels-Alder reactions, e.g. with maleic anhydride to give tetrahydrophthalic anhydride. Like other dienes, butadiene is a ligand for low-valent metal complexes, e.g. the derivatives Fe(butadiene)(CO)3 and Mo(butadiene)3.Environmental health and safety
Butadiene is of low acute toxicity. LC50 is 12.5–11.5 vol% for inhalation by rats and mice. Long-term exposure has been associated with cardiovascular disease. There is a consistent association with leukemia, as well as a significant association with other cancers. IARC has designated 1,3-Butadiene as aGroup 1 Group 1 may refer to:
* Alkali metal, a chemical element classification for Alkali metal
* Group 1 (racing)
The Group 1 racing class referred to FIA regulations for cars in touring car racing and rallying. Throughout its existence the group reta ...
carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substa ...
('carcinogenic to humans'), and the Agency for Toxic Substances Disease Registry and the US EPA also list the chemical as a carcinogen.Health Effects https://www.osha.gov/SLTC/butadiene/index.html The American Conference of Governmental Industrial Hygienists (ACGIH) lists the chemical as a suspected carcinogen. The Natural Resource Defense Council (NRDC) lists some disease clusters that are suspected to be associated with this chemical. Some researchers have concluded it is the most potent carcinogen in cigarette smoke, twice as potent as the runner up acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular st ...
1,3-Butadiene is also a suspected human teratogen. Prolonged and excessive exposure can affect many areas in the human body; blood, brain, eye, heart, kidney, lung, nose and throat have all been shown to react to the presence of excessive 1,3-butadiene. Animal data suggest that women have a higher sensitivity to possible carcinogenic effects of butadiene over men when exposed to the chemical. This may be due to estrogen receptor impacts. While these data reveal important implications to the risks of human exposure to butadiene, more data are necessary to draw conclusive risk assessments. There is also a lack of human data for the effects of butadiene on reproductive and development shown to occur in mice, but animal studies have shown breathing butadiene during pregnancy can increase the number of birth defects, and humans have the same hormone systems as animals./ref> 1,3-Butadiene is recognized as a Highly Reactive Volatile Organic Compound (HRVOC) for its potential to readily form ozone, and as such, emissions of the chemical are highly regulated by
TCEQ
The Texas Commission on Environmental Quality (TCEQ) is the environmental agency for the state of Texas. The commission's headquarters are located at 12100 Park 35 Circle in Austin. The fourth largest environmental agency in the United States (a ...
in parts of the Houston-Brazoria-Galveston Ozone Non-Attainment Areabr>Data sheet
See also
*Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
*Polybutadiene
Polybutadiene utadiene rubber BRis a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tir ...
*Hydroxyl-terminated polybutadiene Hydroxyl-terminated polybutadiene (HTPB) is an oligomer of butadiene terminated at each end with a hydroxyl functional group. It reacts with isocyanates to form polyurethane polymers.
HTPB is a translucent liquid with a color similar to wax paper a ...
References
External links
1,3-Butadiene
– Agency for Toxic Substances and Disease Registry
– CDC - NIOSH Pocket Guide to Chemical Hazards
{{DEFAULTSORT:Butadiene, 1, 3- Alkadienes Hazardous air pollutants Monomers IARC Group 1 carcinogens U.S. Synthetic Rubber Program Commodity chemicals Petrochemicals Conjugated dienes