Bismuth on:
[Wikipedia]
[Google]
[Amazon]
Bismuth is a
 Beginning with Johann Heinrich Pott in 1738,
Beginning with Johann Heinrich Pott in 1738,
 Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of
Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of


 In the Earth's crust, bismuth is about twice as abundant as gold. The most important
In the Earth's crust, bismuth is about twice as abundant as gold. The most important
 The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders,
The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders,
see "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.
 Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
 * Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
*
* Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
*
Lenntech.com. Retrieved on 17 December 2011. As with lead, bismuth poisoning can result in the formation of a black deposit on the
in ''TheFreeDictionary's Medical dictionary''. Farlex, Inc. Poisoning may be treated with
Bismuth
at ''
Bismuth breaks half-life record for alpha decay
{{Authority control Chemical elements Minerals in space group 166 Native element minerals Pnictogens Post-transition metals Alchemical substances Trigonal minerals Materials that expand upon freezing Chemical elements with rhombohedral structure
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol Bi and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
83. It is a post-transition metal
The metallic elements in the periodic table located between the transition metals and the chemically weak nonmetallic metalloids have received many names in the literature, such as ''post-transition metals'', ''poor metals'', ''other metals'', ...
and one of the pnictogen
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the el ...
s, with chemical properties resembling its lighter group 15
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the ...
siblings arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference
Thin-film interference is a natural phenomenon in which light waves reflected by the upper and lower boundaries of a thin film interfere with one another, either enhancing or reducing the reflected light. When the thickness of the film is an ...
. Bismuth is both the most diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
element and one of the least thermally conductive metals known.
Bismuth was long considered the element with the highest atomic mass whose nuclei do not spontaneously decay. However, in 2003 it was discovered to be extremely weakly radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
. The metal's only primordial isotope
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
, bismuth-209
Bismuth-209 (209Bi) is the isotope of bismuth with the longest known half-life of any radioisotope that undergoes α-decay (alpha decay). It has 83 protons and a magic number of 126 neutrons, and an atomic mass of 208.9803987 amu (atomic mass un ...
, experiences alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
at such a minute rate that its half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
is more than a billion times the estimated age of the universe
In physical cosmology, the age of the universe is the time elapsed since the Big Bang. Astronomers have derived two different measurements of the age of the universe:
a measurement based on direct observations of an early state of the universe, ...
. For all but the most exotic of uses bismuth may be considered stable because of its tremendously long half-life.
Bismuth metal has been known since ancient times. Before modern analytical methods bismuth's metallurgical similarities to lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
and tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
often led it to be confused with those metals. The etymology of "bismuth" is uncertain. The name may come from mid- sixteenth century New Latin
New Latin (also called Neo-Latin or Modern Latin) is the revival of Literary Latin used in original, scholarly, and scientific works since about 1500. Modern scholarly and technical nomenclature, such as in zoological and botanical taxonomy ...
translations of the German words or , meaning 'white mass', which were rendered as or .
Main uses
Bismuth compounds account for about half the global production of bismuth. They are used in cosmetics; pigments; and a few pharmaceuticals, notablybismuth subsalicylate
Bismuth subsalicylate, sold generically as pink bismuth and under the brand names Pepto-Bismol and BisBacter, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as nausea, heartburn, indig ...
, used to treat diarrhea
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin w ...
. Bismuth's unusual propensity to expand as it solidifies is responsible for some of its uses, as in the casting of printing type. Bismuth has unusually low toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
for a heavy metal. As the toxicity of lead and the cost of its environmental remediation became more apparent during the 20th century, suitable bismuth alloys have gained popularity as replacements for lead. Presently, around a third of global bismuth production is dedicated to needs formerly met by lead.
History and etymology
Bismuth metal has been known since ancient times and it was one of the first 10 metals to have been discovered. The name ''bismuth'' dates to around 1665 and is of uncertain etymology. The name possibly comes from obsolete German ', ', ' (early 16th century), perhaps related toOld High German
Old High German (OHG; german: Althochdeutsch (Ahd.)) is the earliest stage of the German language, conventionally covering the period from around 750 to 1050.
There is no standardised or supra-regional form of German at this period, and Old High ...
' ("white"). The New Latin
New Latin (also called Neo-Latin or Modern Latin) is the revival of Literary Latin used in original, scholarly, and scientific works since about 1500. Modern scholarly and technical nomenclature, such as in zoological and botanical taxonomy ...
' (coined by Georgius Agricola
Georgius Agricola (; born Georg Pawer or Georg Bauer; 24 March 1494 – 21 November 1555) was a German Humanist scholar, mineralogist and metallurgist. Born in the small town of Glauchau, in the Electorate of Saxony of the Holy Roman Empire ...
, who Latinized many German mining and technical words) is from the German ', itself perhaps from ', meaning "white mass".
The element was confused in early times with tin and lead because of its resemblance to those elements. Because bismuth has been known since ancient times, no one person is credited with its discovery. Agricola
Agricola, the Latin word for farmer, may also refer to:
People Cognomen or given name
:''In chronological order''
* Gnaeus Julius Agricola (40–93), Roman governor of Britannia (AD 77–85)
* Sextus Calpurnius Agricola, Roman governor of the mi ...
(1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties.
Miners in the age of alchemy also gave bismuth the name '','' or "silver being made" in the sense of silver still in the process of being formed within the Earth.
Bismuth was also known to the Incas
The Inca Empire (also known as the Incan Empire and the Inka Empire), called ''Tawantinsuyu'' by its subjects, ( Quechua for the "Realm of the Four Parts", "four parts together" ) was the largest empire in pre-Columbian America. The adm ...
and used (along with the usual copper and tin) in a special bronze alloy for knives.
Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hyd ...
, and Torbern Olof Bergman, the distinctness of lead and bismuth became clear, and Claude François Geoffroy demonstrated in 1753 that this metal is distinct from lead and tin.
Characteristics

Physical characteristics
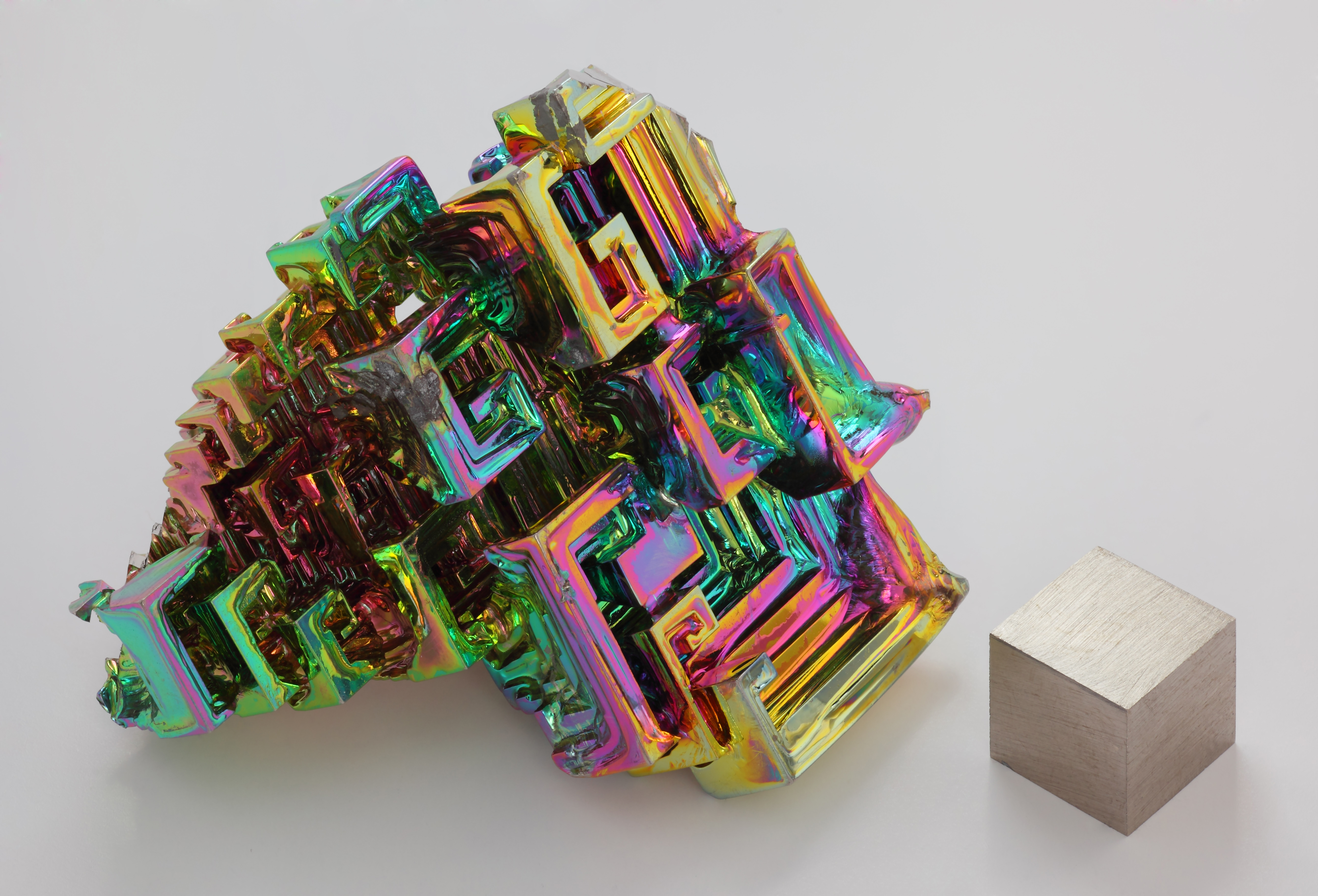
Bismuth is a brittle metal with a dark, silver-pink hue, often with an iridescent oxide tarnish showing many colors from yellow to blue. The spiral, stair-stepped structure of bismuth crystals is the result of a higher growth rate around the outside edges than on the inside edges. The variations in the thickness of the oxide layer that forms on the surface of the crystal cause different wavelengths of light to interfere upon reflection, thus displaying a rainbow of colors. When burned inoxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, bismuth burns with a blue flame
A flame (from Latin '' flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they ...
and its oxide forms yellow fumes. Its toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
is much lower than that of its neighbors in the periodic table, such as lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
.
No other metal is verified to be more naturally diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
than bismuth. ( Superdiamagnetism is a different physical phenomenon.) Of any metal, it has one of the lowest values of thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
(after manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
, and maybe neptunium
Neptunium is a chemical element with the symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it bein ...
and plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
) and the highest Hall coefficient
The Hall effect is the production of a voltage difference (the Hall voltage) across an electrical conductor that is transverse to an electric current in the conductor and to an applied magnetic field perpendicular to the current. It was discov ...
. It has a high electrical resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
. When deposited in sufficiently thin layers on a substrate, bismuth is a semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
, despite being a post-transition metal
The metallic elements in the periodic table located between the transition metals and the chemically weak nonmetallic metalloids have received many names in the literature, such as ''post-transition metals'', ''poor metals'', ''other metals'', ...
. Elemental bismuth is denser in the liquid phase than the solid, a characteristic it shares with germanium, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
, gallium, and water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
. Wiberg, p. 768. Bismuth expands 3.32% on solidification; therefore, it was long a component of low-melting typesetting
Typesetting is the composition of text by means of arranging physical ''type'' (or ''sort'') in mechanical systems or '' glyphs'' in digital systems representing '' characters'' (letters and other symbols).Dictionary.com Unabridged. Random ...
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s, where it compensated for the contraction of the other alloying components to form almost isostatic bismuth-lead eutectic alloys.
Though virtually unseen in nature, high-purity bismuth can form distinctive, colorful hopper crystal
A hopper crystal is a form of crystal, the shape of which resembles that of a pyramidal hopper container.
The edges of hopper crystals are fully developed, but the interior spaces are not filled in. This results in what appears to be a hollowed ...
s. It is relatively nontoxic and has a low melting point just above 271 °C, so crystals may be grown using a household stove, although the resulting crystals will tend to be of lower quality than lab-grown crystals.
At ambient conditions, bismuth shares the same layered structure as the metallic forms of arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
, Wiberg, p. 767. crystallizing in the rhombohedral lattice ( Pearson symbol hR6, space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it uncha ...
Rm No. 166) of the trigonal crystal system. When compressed at room temperature, this Bi-I structure changes first to the monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic s ...
Bi-II at 2.55 GPa, then to the tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a squar ...
Bi-III at 2.7 GPa, and finally to the body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
Bi-V at 7.7 GPa. The corresponding transitions can be monitored via changes in electrical conductivity; they are rather reproducible and abrupt and are therefore used for calibration of high-pressure equipment.
Chemical characteristics
Bismuth is stable to both dry and moist air at ordinary temperatures. When red-hot, it reacts with water to make bismuth(III) oxide. : 2 Bi + 3 H2O → Bi2O3 + 3 H2 It reacts with fluorine to makebismuth(V) fluoride
Bismuth pentafluoride is an inorganic compound with the formula BiF5. It is a white solid that is highly reactive. The compound is of interest to researchers but not of particular value.
Structure
BiF5 is polymeric and consists of linear chains ...
at 500 °C or bismuth(III) fluoride
Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649 °C.
It occurs in nature as the rare mineral gananite.
Synthesis
Bismuth flu ...
at lower temperatures (typically from Bi melts); with other halogens it yields only bismuth(III) halides. Wiberg, pp. 769–770. Greenwood, pp. 559–561. The trihalides are corrosive and easily react with moisture, forming oxyhalides with the formula BiOX. Suzuki, p. 9.
: 4 Bi + 6 X2 → 4 BiX3 (X = F, Cl, Br, I)
: 4 BiX3 + 2 O2 → 4 BiOX + 4 X2
Bismuth dissolves in concentrated sulfuric acid to make bismuth(III) sulfate and sulfur dioxide. Suzuki, p. 8.
: 6 H2SO4 + 2 Bi → 6 H2O + Bi2(SO4)3 + 3 SO2
It reacts with nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
to make bismuth(III) nitrate (which decomposes into nitrogen dioxide
Nitrogen dioxide is a chemical compound with the formula . It is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the productio ...
when heated).
: Bi + 6 HNO3 → 3 H2O + 3 NO2 + Bi(NO3)3
It also dissolves in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
, but only with oxygen present.
: 4 Bi + 3 O2 + 12 HCl → 4 BiCl3 + 6 H2O
It is used as a transmetalating agent in the synthesis of alkaline-earth metal complexes:
: 3 Ba + 2 BiPh3 → 3 BaPh2 + 2 Bi
Isotopes
The only primordialisotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
of bismuth, bismuth-209
Bismuth-209 (209Bi) is the isotope of bismuth with the longest known half-life of any radioisotope that undergoes α-decay (alpha decay). It has 83 protons and a magic number of 126 neutrons, and an atomic mass of 208.9803987 amu (atomic mass un ...
, was traditionally regarded as the heaviest stable isotope, but it had long been suspected to be unstable on theoretical grounds. This was finally demonstrated in 2003, when researchers at the '' Institut d'Astrophysique Spatiale'' in Orsay
Orsay () is a commune in the Essonne department in Île-de-France in northern France. It is located in the southwestern suburbs of Paris, France, from the centre of Paris.
A fortified location of the Chevreuse valley since the 8th centur ...
, France, measured the alpha emission
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of to be (3 Bq/ M g), over a billion
Billion is a word for a large number, and it has two distinct definitions:
*1,000,000,000, i.e. one thousand million, or (ten to the ninth power), as defined on the short scale. This is its only current meaning in English.
* 1,000,000,000,000, i. ...
times longer than the current estimated age of the universe
In physical cosmology, the age of the universe is the time elapsed since the Big Bang. Astronomers have derived two different measurements of the age of the universe:
a measurement based on direct observations of an early state of the universe, ...
. Owing to its extraordinarily long half-life, for all presently known medical and industrial applications, bismuth can be treated as if it is stable and nonradioactive. The radioactivity is of academic interest because bismuth is one of a few elements whose radioactivity was suspected and theoretically predicted before being detected in the laboratory. Bismuth has the longest known alpha decay half-life, although tellurium-128 has a double beta decay
In nuclear physics, double beta decay is a type of radioactive decay in which two neutrons are simultaneously transformed into two protons, or vice versa, inside an atomic nucleus. As in single beta decay, this process allows the atom to move clos ...
half-life of over . Bismuth's extremely long half-life means that less than approximately one-billionth of the bismuth present at the formation of the planet Earth would have decayed into thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
since then.
Several isotopes of bismuth with short half-lives occur within the radioactive disintegration chains of actinium
Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance An ...
, radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
, and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
, and more have been synthesized experimentally. Bismuth-213 is also found on the decay chain of neptunium-237
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be sy ...
and uranium-233
Uranium-233 (233U or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a reactor fuel. It has been used successfully in exp ...
.
Commercially, the radioactive isotope bismuth-213 can be produced by bombarding radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rathe ...
with bremsstrahlung
''Bremsstrahlung'' (), from "to brake" and "radiation"; i.e., "braking radiation" or "deceleration radiation", is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typicall ...
photons from a linear particle accelerator
A linear particle accelerator (often shortened to linac) is a type of particle accelerator that accelerates charged subatomic particles or ions to a high speed by subjecting them to a series of oscillating electric potentials along a linear b ...
. In 1997, an antibody conjugate with bismuth-213, which has a 45-minute half-life and decays with the emission of an alpha particle, was used to treat patients with leukemia. This isotope has also been tried in cancer treatment, for example, in the targeted alpha therapy (TAT) program.
Chemical compounds
 Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of
Bismuth forms trivalent and pentavalent compounds, the trivalent ones being more common. Many of its chemical properties are similar to those of arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
, although they are less toxic than derivatives of those lighter elements.
Oxides and sulfides
At elevated temperatures, the vapors of the metal combine rapidly with oxygen, forming the yellow trioxide, . Greenwood, p. 553. When molten, at temperatures above 710 °C, this oxide corrodes any metal oxide and even platinum. Krüger, p. 185 On reaction with a base, it forms two series ofoxyanion An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determine ...
s: , which is polymeric and forms linear chains, and . The anion in is a cubic octameric anion, , whereas the anion in is tetrameric.
The dark red bismuth(V) oxide, , is unstable, liberating gas upon heating. The compound NaBiO3 is a strong oxidising agent. Greenwood, p. 578.
Bismuth sulfide, , occurs naturally in bismuth ores. It is also produced by the combination of molten bismuth and sulfur.

Bismuth oxychloride
Bismuth oxychloride is an inorganic compound of bismuth with the formula Bi O Cl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light ...
(BiOCl, see figure at right) and bismuth oxynitrate (BiONO3) stoichiometrically appear as simple anionic salts of the bismuthyl(III) cation (BiO+) which commonly occurs in aqueous bismuth compounds. However, in the case of BiOCl, the salt crystal forms in a structure of alternating plates of Bi, O, and Cl atoms, with each oxygen coordinating with four bismuth atoms in the adjacent plane. This mineral compound is used as a pigment and cosmetic (see below).
Bismuthine and bismuthides
Unlike the lighterpnictogen
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the el ...
s nitrogen, phosphorus, and arsenic, but similar to antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient t ...
, bismuth does not form a stable hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
. Bismuth hydride, bismuthine (), is an endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
compound that spontaneously decomposes at room temperature. It is stable only below −60 °C. Bismuthides are intermetallic
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
compounds between bismuth and other metals.
In 2014 researchers discovered that sodium bismuthide can exist as a form of matter called a “three-dimensional topological Dirac semi-metal” (3DTDS) that possess 3D Dirac fermions in bulk. It is a natural, three-dimensional counterpart to graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
with similar electron mobility and velocity. Graphene and topological insulator
A topological insulator is a material whose interior behaves as an electrical insulator while its surface behaves as an electrical conductor, meaning that electrons can only move along the surface of the material.
A topological insulator is an ...
s (such as those in 3DTDS) are both crystalline materials that are electrically insulating inside but conducting on the surface, allowing them to function as transistor
upright=1.4, gate (G), body (B), source (S) and drain (D) terminals. The gate is separated from the body by an insulating layer (pink).
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch ...
s and other electronic devices. While sodium bismuthide () is too unstable to be used in devices without packaging, it can demonstrate potential applications of 3DTDS systems, which offer distinct efficiency and fabrication advantages over planar graphene in semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
and spintronics
Spintronics (a portmanteau meaning spin transport electronics), also known as spin electronics, is the study of the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid-st ...
applications.
Halides
The halides of bismuth in low oxidation states have been shown to adopt unusual structures. What was originally thought to be bismuth(I) chloride, BiCl, turns out to be a complex compound consisting of Bi cations and BiCl and BiCl anions. The Bi cation has a distorted tricappedtrigonal prism
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The oc ...
atic molecular geometry and is also found in , which is prepared by reducing a mixture of hafnium(IV) chloride and bismuth chloride with elemental bismuth, having the structure . Other polyatomic bismuth cations are also known, such as Bi, found in . Bismuth also forms a low-valence bromide with the same structure as "BiCl". There is a ''true'' monoiodide, BiI, which contains chains of units. BiI decomposes upon heating to the triiodide, , and elemental bismuth. A monobromide of the same structure also exists.
In oxidation state +3, bismuth forms trihalides with all of the halogens: , , , and . All of these except are hydrolyze
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
d by water.
Bismuth(III) chloride
Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a covalent compound and is the common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, i ...
reacts with hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
in ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
solution to produce the acid .
The oxidation state +5 is less frequently encountered. One such compound is , a powerful oxidizing and fluorinating agent. It is also a strong fluoride acceptor, reacting with xenon tetrafluoride to form the cation:
: + →
Aqueous species
Inaqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
solution, the Bi ion is solvated to form the aqua ion in strongly acidic conditions. At pH > 0 polynuclear species exist, the most important of which is believed to be the octahedral complex [].
Occurrence and production

 In the Earth's crust, bismuth is about twice as abundant as gold. The most important
In the Earth's crust, bismuth is about twice as abundant as gold. The most important ore
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit.Encyclopædia Britannica. "Ore". Encyclopædia Britannica Online. Retrieved 7 Apr ...
s of bismuth are bismuthinite
Bismuthinite is a mineral consisting of bismuth(III) sulfide, bismuth sulfide (bismuth, Bi2sulfur, S3). It is an important ore for bismuth. The crystals are steel-grey to off-white with a metallic luster. It is soft enough to be scratched with ...
and bismite
Bismite is a bismuth oxide mineral, bismuth trioxide or Bi2O3. It is a monoclinic mineral, but the typical form of occurrence is massive and clay-like with no macroscopic crystals. The color varies from green to yellow. It has a Mohs hardness of ...
. Native bismuth is known from Australia, Bolivia, and China.
The difference between mining and refining production reflects bismuth's status as a byproduct of extraction of other metals such as lead, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
, molybdenum and tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
. World bismuth production from refineries is a more complete and reliable statistic.
Bismuth travels in crude lead bullion (which can contain up to 10% bismuth) through several stages of refining, until it is removed by the Kroll-Betterton process which separates the impurities as slag, or the electrolytic Betts process. Bismuth will behave similarly with another of its major metals, copper. The raw bismuth metal from both processes contains still considerable amounts of other metals, foremost lead. By reacting the molten mixture with chlorine gas the metals are converted to their chlorides while bismuth remains unchanged. Impurities can also be removed by various other methods for example with fluxes and treatments yielding high-purity bismuth metal (over 99% Bi).
Price
 The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders,
The price for pure bismuth metal has been relatively stable through most of the 20th century, except for a spike in the 1970s. Bismuth has always been produced mainly as a byproduct of lead refining, and thus the price usually reflected the cost of recovery and the balance between production and demand.
Prior to World War II, demand for bismuth was small and mainly pharmaceutical — bismuth compounds were used to treat such conditions as digestive disorders, sexually transmitted diseases
Sexually transmitted infections (STIs), also referred to as sexually transmitted diseases (STDs) and the older term venereal diseases, are infections that are spread by sexual activity, especially vaginal intercourse, anal sex, and oral ...
and burns. Minor amounts of bismuth metal were consumed in fusible alloys for fire sprinkler
A fire sprinkler or sprinkler head is the component of a fire sprinkler system that discharges water when the effects of a fire have been detected, such as when a predetermined temperature has been exceeded. Fire sprinklers are extensively use ...
systems and fuse wire
In electronics and electrical engineering, a fuse is an electrical safety device that operates to provide overcurrent protection of an electrical circuit. Its essential component is a metal wire or strip that melts when too much current flows thro ...
. During World War II bismuth was considered a strategic material, used for solders, fusible alloys, medications and atomic research. To stabilize the market, the producers set the price at $1.25 per pound ($2.75 /kg) during the war and at $2.25 per pound ($4.96 /kg) from 1950 until 1964.
In the early 1970s, the price rose rapidly as a result of increasing demand for bismuth as a metallurgical additive to aluminium, iron and steel. This was followed by a decline owing to increased world production, stabilized consumption, and the recessions of 1980 and 1981–1982. In 1984, the price began to climb as consumption increased worldwide, especially in the United States and Japan. In the early 1990s, research began on the evaluation of bismuth as a nontoxic replacement for lead in ceramic glazes, fishing sinkers, food-processing equipment, free-machining brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other wit ...
es for plumbing applications, lubricating greases, and shot for waterfowl hunting
Waterfowl hunting (also called wildfowling or waterfowl shooting in the UK) is the practice of hunting ducks, geese, or other waterfowl for food and sport.
Many types of ducks and geese share the same habitat, have overlapping or identical hunt ...
. Suzuki, p. 14. Growth in these areas remained slow during the middle 1990s, in spite of the backing of lead replacement by the United States federal government, but intensified around 2005. This resulted in a rapid and continuing increase in price.Bismuth Statistics and Informationsee "Metal Prices in the United States through 1998" for a price summary and "Historical Statistics for Mineral and Material Commodities in the United States" for production. USGS.
Recycling
Most bismuth is produced as a byproduct of other metal-extraction processes including the smelting of lead, and also of tungsten and copper. Its sustainability is dependent on increased recycling, which is problematic. It was once believed that bismuth could be practically recycled from the soldered joints in electronic equipment. Recent efficiencies in solder application in electronics mean there is substantially less solder deposited, and thus less to recycle. While recovering the silver from silver-bearing solder may remain economic, recovering bismuth is substantially less so. Next in recycling feasibility would be sizeable catalysts with a fair bismuth content, such as bismuth phosphomolybdate. Bismuth used in galvanizing, and as a free-machining metallurgical additive. Bismuth in uses where it is dispersed most widely include certain stomach medicines (bismuth subsalicylate
Bismuth subsalicylate, sold generically as pink bismuth and under the brand names Pepto-Bismol and BisBacter, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as nausea, heartburn, indig ...
), paints ( bismuth vanadate), pearlescent cosmetics (bismuth oxychloride
Bismuth oxychloride is an inorganic compound of bismuth with the formula Bi O Cl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light ...
), and bismuth-containing bullets. Recycling bismuth from these uses is impractical.
Applications
 Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 733 tonnes of bismuth were consumed in 2016, of which 70% went into chemicals (including pharmaceuticals, pigments, and cosmetics) and 11% into bismuth alloys.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet "lead-free" mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
In the early 1990s, researchers began to evaluate bismuth as a nontoxic replacement for lead in various applications.
Medicines
Bismuth is an ingredient in some pharmaceuticals, although the use of some of these substances is declining. *Bismuth subsalicylate
Bismuth subsalicylate, sold generically as pink bismuth and under the brand names Pepto-Bismol and BisBacter, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as nausea, heartburn, indig ...
is used as an antidiarrheal
An anti-diarrhoeal drug (or anti-diarrheal drug in American English) is any medication which provides symptomatic relief for diarrhoea.
Types
* Electrolyte solutions, while not true antidiarrhoeals, are used to replace lost fluids and salts in ac ...
; it is the active ingredient
An active ingredient is any ingredient that provides biologically active or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the body of humans or animals. The ...
in such "pink bismuth" preparations as Pepto-Bismol
Bismuth subsalicylate, sold generically as pink bismuth and under the brand names Pepto-Bismol and BisBacter, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as nausea, heartburn, indig ...
, as well as the 2004 reformulation of Kaopectate
Kaopectate is an orally taken medication from Arcadia Consumer Healthcare for the treatment of mild diarrhea. It is also sometimes used to treat indigestion, nausea, and stomach ulcers. The active ingredients have varied over time, and are differ ...
. It is also used to treat some other gastro-intestinal diseases like shigellosis and cadmium poisoning
Cadmium is a naturally occurring toxic metal with common exposure in industrial workplaces, plant soils, and from smoking. Due to its low permissible exposure in humans, overexposure may occur even in situations where trace quantities of cadmium ...
. The mechanism of action of this substance is still not well documented, although an oligodynamic effect (toxic effect of small doses of heavy metal ions on microbes) may be involved in at least some cases. Salicylic acid from hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
of the compound is antimicrobial for toxogenic ''E. coli,'' an important pathogen in traveler's diarrhea
Travelers' diarrhea (TD) is a stomach and intestinal infection. TD is defined as the passage of unformed stool (one or more by some definitions, three or more by others) while traveling. It may be accompanied by abdominal cramps, nausea, fever, ...
.
* A combination of bismuth subsalicylate
Bismuth subsalicylate, sold generically as pink bismuth and under the brand names Pepto-Bismol and BisBacter, is an antacid medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as nausea, heartburn, indig ...
and bismuth subcitrate is used to treat the bacteria causing peptic ulcer
Peptic ulcer disease (PUD) is a break in the inner lining of the stomach, the first part of the small intestine, or sometimes the lower esophagus. An ulcer in the stomach is called a gastric ulcer, while one in the first part of the intestines ...
s.
* Bibrocathol
Bibrocathol (International Nonproprietary Name, INN, trade names Noviform and Posiformin) is the substance 4,5,6,7-Tetrabrom-1,3,2-benzodioxabismol-2-ol. It contains bismuth and is used to treat eye infections and control swelling.
References
...
is an organic bismuth-containing compound used to treat eye infections.
* Bismuth subgallate
Bismuth subgallate, with a chemical formula C7H5BiO6, is commonly used to treat malodor by deodorizing flatulence and stools. In the United States, it (bismuth subgallate) is the active ingredient in Devrom (internal deodorant), an over-the-coun ...
, the active ingredient
An active ingredient is any ingredient that provides biologically active or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the body of humans or animals. The ...
in Devrom, is used as an internal deodorant to treat malodor from flatulence and feces.
* Bismuth compounds (including sodium bismuth tartrate) were formerly used to treat syphilis. Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, ...
combined with either bismuth or mercury was a mainstay of syphilis treatment from the 1920s until the advent of penicillin in 1943.
* "Milk of bismuth" (an aqueous suspension of bismuth hydroxide
Bismuth hydroxide () is non-fully characterised chemical compound of bismuth. It is produced as white flakes when alkali is added to a solution of a bismuth salt and is usually described as bismuth oxide hydrate or bismuth hydrate.
Uses
Bismuth h ...
and bismuth subcarbonate
Bismuth subcarbonate (BiO)2CO3, sometimes written Bi2O2(CO3) is a chemical compound of bismuth containing both oxide and carbonate anions. Bismuth is in the +3 oxidation state. Bismuth subcarbonate occurs naturally as the mineral bismutite. Its s ...
) was marketed as an alimentary cure-all in the early 20th century, and has been used to treat gastrointestinal disorders
Gastrointestinal diseases (abbrev. GI diseases or GI illnesses) refer to diseases involving the gastrointestinal tract, namely the oesophagus, stomach, small intestine, large intestine and rectum, and the accessory organs of digestion, the live ...
.
* Bismuth subnitrate
Bismuth oxynitrate is the name applied to a number of compounds that contain bismuth, Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth ...
(Bi5O(OH)9(NO3)4) and bismuth subcarbonate
Bismuth subcarbonate (BiO)2CO3, sometimes written Bi2O2(CO3) is a chemical compound of bismuth containing both oxide and carbonate anions. Bismuth is in the +3 oxidation state. Bismuth subcarbonate occurs naturally as the mineral bismutite. Its s ...
(Bi2O2(CO3)) are also used in medicine.
Cosmetics and pigments
Bismuth oxychloride
Bismuth oxychloride is an inorganic compound of bismuth with the formula Bi O Cl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light ...
(BiOCl) is sometimes used in cosmetics, as a pigment in paint for eye shadows, hair sprays and nail polishes. This compound is found as the mineral bismoclite and in crystal form contains layers of atoms (see figure above) that refract light chromatically, resulting in an iridescent appearance similar to nacre of pearl. It was used as a cosmetic in ancient Egypt and in many places since. ''Bismuth white'' (also "Spanish white") can refer to either bismuth oxychloride or bismuth oxynitrate (BiONO3), when used as a white pigment. Bismuth vanadate is used as a light-stable non-reactive paint pigment (particularly for artists' paints), often as a replacement for the more toxic cadmium sulfide yellow and orange-yellow pigments. The most common variety in artists' paints is a lemon yellow, visually indistinguishable from its cadmium-containing alternative.
Metal and alloys
Bismuth is used in metal alloys with other metals such as iron. These alloys are used in automatic sprinkler systems for fires. It forms the largest part (50%) of Rose's metal, afusible alloy A fusible alloy is a metal alloy capable of being easily fused, i.e. easily meltable, at relatively low temperatures. Fusible alloys are commonly, but not necessarily, eutectic alloys.
Sometimes the term "fusible alloy" is used to describe alloys ...
, which also contains 25–28% lead and 22–25% tin. It was also used to make bismuth bronze which was used in the Bronze Age
The Bronze Age is a historic period, lasting approximately from 3300 BC to 1200 BC, characterized by the use of bronze, the presence of writing in some areas, and other early features of urban civilization. The Bronze Age is the second prin ...
, having been found in Inca knives at Machu Picchu.
Lead replacement
The density difference between lead (11.32 g/cm3) and bismuth (9.78 g/cm3) is small enough that for many ballistics and weighting applications, bismuth can substitute forlead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
. For example, it can replace lead as a dense material in fishing sinker
A fishing sinker or knoch is a weight used in conjunction with a fishing lure or hook to increase its rate of sink, anchoring ability, and/or casting distance. Fishing sinkers may be as small as 1 gram for applications in shallow water, and even ...
s. It has been used as a replacement for lead in shot, bullets and less-lethal
Non-lethal weapons, also called nonlethal weapons, less-lethal weapons, less-than-lethal weapons, non-deadly weapons, compliance weapons, or pain-inducing weapons are weapons intended to be less likely to kill a living target than conventional ...
riot gun
In current usage, a riot gun or less-lethal launcher is a type of firearm used to fire "non-lethal" or "less-lethal" ammunition for the purpose of suppressing riots or apprehending suspects with minimal harm or risk. Less-lethal launchers may ...
ammunition. The Netherlands, Denmark, England, Wales, the United States, and many other countries now prohibit the use of lead shot for the hunting of wetland birds, as many birds are prone to lead poisoning owing to mistaken ingestion of lead (instead of small stones and grit) to aid digestion, or even prohibit the use of lead for all hunting, such as in the Netherlands. Bismuth-tin alloy shot is one alternative that provides similar ballistic performance to lead. (Another less expensive but also more poorly performing alternative is "steel" shot, which is actually soft iron.) Bismuth's lack of malleability does, however, make it unsuitable for use in expanding hunting bullets.
Bismuth, as a dense element of high atomic weight, is used in bismuth-impregnated latex shields to shield from X-ray in medical examinations, such as CTs, mostly as it is considered non-toxic.
The European Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been de ...
's Restriction of Hazardous Substances Directive
The Restriction of Hazardous Substances Directive 2002/95/EC (RoHS 1), short for Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment, was adopted in February 2003 by the European Unio ...
(RoHS) for reduction of lead has broadened bismuth's use in electronics as a component of low-melting point solders, as a replacement for traditional tin-lead solders. Its low toxicity will be especially important for solders to be used in food processing equipment and copper water pipes, although it can also be used in other applications including those in the automobile industry, in the European Union, for example.
Bismuth has been evaluated as a replacement for lead in free-machining brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other wit ...
es for plumbing
Plumbing is any system that conveys fluids for a wide range of applications. Plumbing uses pipes, valves, plumbing fixtures, tanks, and other apparatuses to convey fluids. Heating and cooling (HVAC), waste removal, and potable water deliv ...
applications, although it does not equal the performance of leaded steels.
Other metal uses and specialty alloys
Many bismuthalloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s have low melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
s and are found in specialty applications such as solder
Solder (; NA: ) is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable ...
s. Many automatic sprinklers, electric fuses, and safety devices in fire detection and suppression systems contain the eutectic In19.1-Cd5.3-Pb22.6-Sn8.3-Bi44.7 alloy that melts at This is a convenient temperature since it is unlikely to be exceeded in normal living conditions. Low-melting alloys, such as Bi-Cd-Pb-Sn alloy which melts at 70 °C, are also used in automotive and aviation industries. Before deforming a thin-walled metal part, it is filled with a melt or covered with a thin layer of the alloy to reduce the chance of breaking. Then the alloy is removed by submerging the part in boiling water. Krüger, p. 183.
Bismuth is used to make free-machining steels and free-machining aluminium alloys for precision machining properties. It has similar effect to lead and improves the chip breaking during machining. The shrinking on solidification in lead and the expansion of bismuth compensate each other and therefore lead and bismuth are often used in similar quantities. Similarly, alloys containing comparable parts of bismuth and lead exhibit a very small change (on the order 0.01%) upon melting, solidification or aging. Such alloys are used in high-precision casting, e.g. in dentistry, to create models and molds. Bismuth is also used as an alloying agent in production of malleable irons and as a thermocouple
A thermocouple, also known as a "thermoelectrical thermometer", is an electrical device consisting of two dissimilar electrical conductors forming an electrical junction. A thermocouple produces a temperature-dependent voltage as a result of th ...
material.
Bismuth is also used in aluminium-silicon cast alloys in order to refine silicon morphology. However, it indicated a poisoning effect on modification of strontium. Some bismuth alloys, such as Bi35-Pb37-Sn25, are combined with non-sticking materials such as mica, glass and enamels because they easily wet them allowing to make joints to other parts. Addition of bismuth to caesium enhances the quantum yield of caesium cathodes. Krüger, p. 184. Sintering
Clinker nodules produced by sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction.
Sintering happens as part of a manufacturing ...
of bismuth and manganese powders at 300 °C produces a permanent magnet and magnetostrictive material, which is used in ultrasonic generators and receivers working in the 10–100 kHz range and in magnetic and holographic
Holography is a technique that enables a wavefront to be recorded and later re-constructed. Holography is best known as a method of generating real three-dimensional images, but it also has a wide range of other applications. In principle, i ...
memory devices. Suzuki, p. 15.
Other uses as compounds
 * Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
*
* Bismuth is included in BSCCO (bismuth strontium calcium copper oxide) which is a group of similar superconducting compounds discovered in 1988 that exhibit the highest superconducting transition temperatures.
* Bismuth subnitrate
Bismuth oxynitrate is the name applied to a number of compounds that contain bismuth, Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth ...
is a component of glazes that produces an iridescence and is used as a pigment in paint.
* Bismuth telluride
Bismuth telluride (Bi2Te3) is a gray powder that is a compound of bismuth and tellurium also known as bismuth(III) telluride. It is a semiconductor, which, when alloyed with antimony or selenium, is an efficient thermoelectric material for refriger ...
is a semiconductor and an excellent thermoelectric material. Bi2Te3 diodes are used in mobile refrigerators, CPU coolers, and as detectors in infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
spectrophotometers.
* Bismuth oxide
Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite (tetragonal, much more rare ...
, in its delta form, is a solid electrolyte for oxygen. This form normally breaks down below a high-temperature threshold, but can be electrodeposited well below this temperature in a highly alkaline solution.
* Bismuth germanate is a scintillator, widely used in X-ray and gamma ray detectors.
* Bismuth vanadate is an opaque yellow pigment used by some artists' oil, acrylic, and watercolor paint companies, primarily as a replacement for the more toxic cadmium sulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid.Egon Wiberg, Arnold Frederick Holleman (2001''Inorganic Chemistry'' Elsevier It occurs in nature with two different crystal structures as the rare mi ...
yellows in the greenish-yellow (lemon) to orange-toned yellow range. It performs practically identically to the cadmium pigments, such as in terms of resistance to degradation from UV exposure, opacity, tinting strength, and lack of reactivity when mixed with other pigments. The most commonly-used variety by artists' paint makers is lemon in color. In addition to being a replacement for several cadmium yellows, it also serves as a non-toxic visual replacement for the older chromate pigments made with zinc, lead, and strontium. If a green pigment and barium sulfate (for increased transparency) are added it can also serve as a replacement for barium chromate
Barium chromate, named barium tetraoxochromate(VI) by the IUPAC, is a yellow sand like powder with the formula BaCrO4. It is a known oxidizing agent and produces a green flame when heated, a result of the barium ions.
History
The first naturally o ...
, which possesses a more greenish cast than the others. In comparison with lead chromate
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, l ...
, it does not blacken due to hydrogen sulfide in the air (a process accelerated by UV exposure) and possesses a particularly brighter color than them, especially the lemon, which is the most translucent, dull, and fastest to blacken due to the higher percentage of lead sulfate required to produce that shade. It is also used, on a limited basis due to its cost, as a vehicle paint pigment.
* A catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
for making acrylic fibers.
* As an electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst ...
in the conversion of CO2 to CO.
* Ingredient in lubricating
Lubrication is the process or technique of using a lubricant to reduce friction and wear and tear in a contact between two surfaces. The study of lubrication is a discipline in the field of tribology.
Lubrication mechanisms such as fluid-lubrica ...
greases.
* In crackling microstars ( dragon's eggs) in pyrotechnics, as the oxide, subcarbonate or subnitrate.
*As catalyst for the fluorination of arylboronic pinacol esters through a Bi(III)/Bi(V) catalytic cycle, mimicking transition metals in electrophilic fluorination.
Toxicology and ecotoxicology
:''See alsobismuthia {{Short description, Medical condition
Bismuthia is a rare dermatological condition that results from the prolonged use of bismuth.
Much more rarely than with silver, bismuth may produce a generalized persistent skin discoloration resembling argyri ...
, a rare dermatological condition that results from the prolonged use of bismuth.''
Scientific literature indicates that some of the compounds of bismuth are less toxic to humans via ingestion than other heavy metals (lead, arsenic, antimony, etc.) presumably due to the comparatively low solubility of bismuth salts. Its biological half-life
Biological half-life (also known as elimination half-life, pharmacologic half-life) is the time taken for concentration of a biological substance (such as a medication) to decrease from its maximum concentration ( Cmax) to half of Cmax in the bl ...
for whole-body retention is reported to be 5 days but it can remain in the kidney for years in people treated with bismuth compounds.
Bismuth poisoning can occur and has according to some reports been common in relatively recent times.Data on Bismuth's health and environmental effectsLenntech.com. Retrieved on 17 December 2011. As with lead, bismuth poisoning can result in the formation of a black deposit on the
gingiva
The gums or gingiva (plural: ''gingivae'') consist of the mucosal tissue that lies over the mandible and maxilla inside the mouth. Gum health and disease can have an effect on general health.
Structure
The gums are part of the soft tissue lin ...
, known as a bismuth line."Bismuth line"in ''TheFreeDictionary's Medical dictionary''. Farlex, Inc. Poisoning may be treated with
dimercaprol
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead. It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is n ...
; however, evidence for benefit is unclear.
Bismuth's environmental impacts are not well known; it may be less likely to bioaccumulate than some other heavy metals, and this is an area of active research.
See also
*Lead-bismuth eutectic
Lead-Bismuth Eutectic or LBE is a eutectic alloy of lead (44.5 at%) and bismuth (55.5 at%) used as a coolant in some nuclear reactors, and is a proposed coolant for the lead-cooled fast reactor, part of the Generation IV reactor initiative.
It h ...
* List of countries by bismuth production
Bismuth is a chemical element that has the symbol Bi and atomic number 83. This heavy, brittle, white crystalline trivalent other metal has a pink tinge and chemically resembles arsenic and antimony.
Of all the metals, it is the most naturally ...
* Bismuth minerals
* Patterns in nature
Patterns in nature are visible regularities of form found in the natural world. These patterns recur in different contexts and can sometimes be modelled mathematically. Natural patterns include symmetries, trees, spirals, meanders, waves, ...
References
Bibliography
* * * *External links
* Laboratory growth of large crystals of Bismuth by Jan Kihle Crystal Pulling Laboratories, NorwayBismuth
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Bismuth breaks half-life record for alpha decay
{{Authority control Chemical elements Minerals in space group 166 Native element minerals Pnictogens Post-transition metals Alchemical substances Trigonal minerals Materials that expand upon freezing Chemical elements with rhombohedral structure