Amyloid fibril on:
[Wikipedia]
[Google]
[Amazon]
Amyloids are aggregates of 
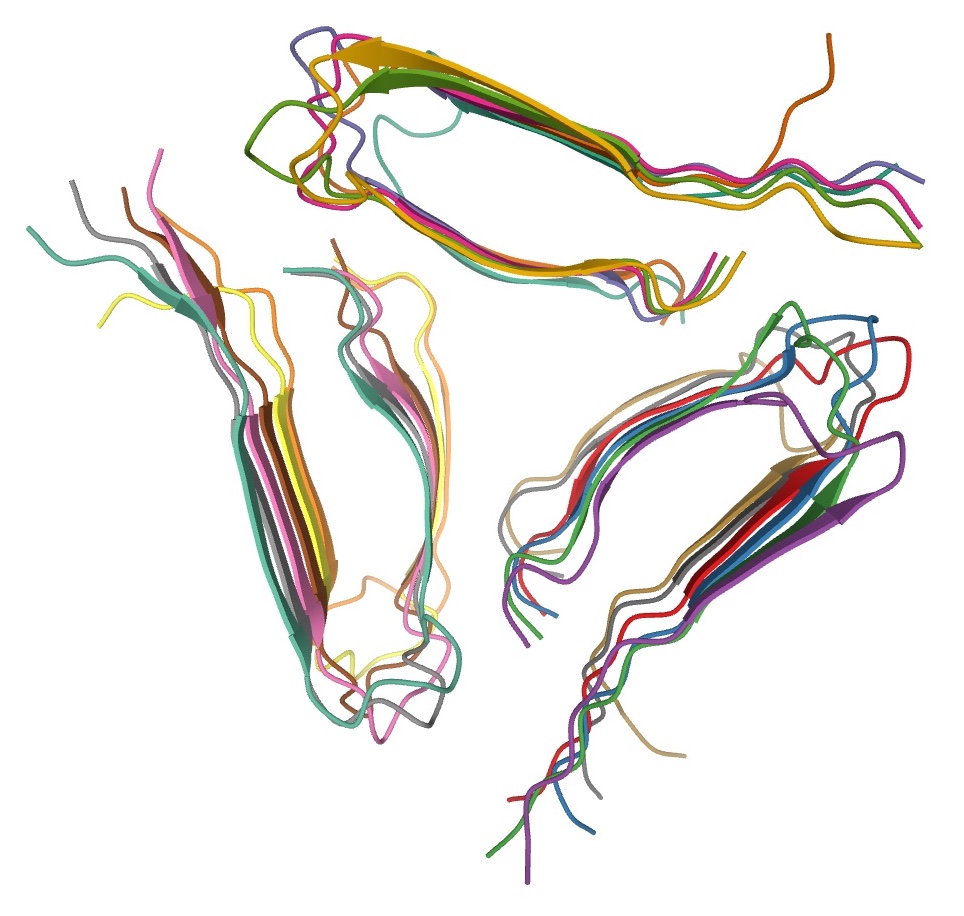 Amyloids are formed of long unbranched fibers that are characterized by an extended beta-sheet secondary structure in which individual
Amyloids are formed of long unbranched fibers that are characterized by an extended beta-sheet secondary structure in which individual
Michaels
and coworkers and considers the time evolution of the concentration of fibrils of length (here represents the number of monomers in an aggregate). where denotes the
Bacterial Inclusion Bodies Contain Amyloid-Like Structure
at SciVee
Amyloid Cascade Hypothesis
Amyloid: Journal of Protein Folding Disorders web page
{{Amyloidosis Amyloidosis Histopathology Structural proteins
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s characterised by a fibrillar morphology of 7–13 nm in diameter
In geometry, a diameter of a circle is any straight line segment that passes through the center of the circle and whose endpoints lie on the circle. It can also be defined as the longest chord of the circle. Both definitions are also valid fo ...
, a beta sheet (β-sheet) secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red
Congo red is an organic compound, the sodium salt of 3,3′-( ,1′-biphenyl4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid). It is an azo dye. Congo red is water-soluble, yielding a red colloidal solution; its solubility is greater in organic ...
. In the human body, amyloids have been linked to the development of various disease
A disease is a particular abnormal condition that negatively affects the structure or function of all or part of an organism, and that is not immediately due to any external injury. Diseases are often known to be medical conditions that a ...
s. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions (misfolding
Protein folding is the physical process by which a protein chain is Translation (biology), translated to its native protein tertiary structure, three-dimensional structure, typically a "folded" Protein structure, conformation by which the prote ...
) and form fibrous deposits in amyloid plaques around cells which can disrupt the healthy function of tissues and organs.
Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are familial. Others are only familial. Some are iatrogenic
Iatrogenesis is the causation of a disease, a harmful complication, or other ill effect by any medical activity, including diagnosis, intervention, error, or negligence. "Iatrogenic", ''Merriam-Webster.com'', Merriam-Webster, Inc., accessed 27 ...
as they result from medical treatment. Prions
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It i ...
are an infectious
An infection is the invasion of tissues by pathogens, their multiplication, and the reaction of host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable dis ...
form of amyloids that can act as a template to convert other non-infectious forms. Amyloids may also have normal biological functions; for example, in the formation of fimbriae in some genera of bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
, transmission of epigenetic traits in fungi, as well as pigment deposition and hormone release in humans.
Amyloids have been known to arise from many different proteins. These polypeptide chains generally form β-sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
structures that aggregate into long fibers; however, identical polypeptides can fold into multiple distinct amyloid conformations. The diversity of the conformations may have led to different forms of the prion diseases.
An unusual secondary structure named alpha sheet
Alpha sheet (also known as alpha pleated sheet or polar pleated sheet) is an atypical secondary structure in proteins, first proposed by Linus Pauling and Robert Corey in 1951.Pauling, L. & Corey, R. B. (1951). The pleated sheet, a new layer con ...
has been proposed as the toxic constituent of amyloid precursor proteins. This idea is not widely accepted at present, but a fair amount of evidence has accumulated, especially recently, in its favor.

Definition
The name ''amyloid'' comes from the early mistaken identification byRudolf Virchow
Rudolf Ludwig Carl Virchow (; or ; 13 October 18215 September 1902) was a German physician, anthropologist, pathologist, prehistorian, biologist, writer, editor, and politician. He is known as "the father of modern pathology" and as the founder ...
of the substance as starch ( in Latin
Latin (, or , ) is a classical language belonging to the Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power of the ...
, from ), based on crude iodine-staining techniques. For a period, the scientific community debated whether or not amyloid deposits are fatty deposits or carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
deposits until it was finally found (in 1859) that they are, in fact, deposits of albumoid proteinaceous material.
* The classical, histopathological
Histopathology (compound of three Greek words: ''histos'' "tissue", πάθος ''pathos'' "suffering", and -λογία ''-logia'' "study of") refers to the microscopic examination of tissue in order to study the manifestations of disease. Spec ...
definition of amyloid is an extracellular, proteinaceous fibrillar deposit exhibiting β-sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
secondary structure and identified by apple-green birefringence when stained with congo red
Congo red is an organic compound, the sodium salt of 3,3′-( ,1′-biphenyl4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid). It is an azo dye. Congo red is water-soluble, yielding a red colloidal solution; its solubility is greater in organic ...
under polarized light. These deposits often recruit various sugars and other components such as serum amyloid P component
The serum amyloid P component (SAP) is the identical serum form of amyloid P component (AP), a 25kDa pentameric protein first identified as the pentagonal constituent of in vivo pathological deposits called "amyloid". APCS is its human gene.
In ...
, resulting in complex, and sometimes inhomogeneous structures. Recently this definition has come into question as some classic, amyloid species have been observed in distinctly intracellular locations.
* A more recent, ''biophysical'' definition is broader, including any polypeptide that polymerizes to form a cross-β structure, ''in vivo'' or ''in vitro'', inside or outside cells. Microbiologists
A microbiologist (from Greek ) is a scientist who studies microscopic life forms and processes. This includes study of the growth, interactions and characteristics of microscopic organisms such as bacteria, algae, fungi, and some types of para ...
, biochemists
Biochemists are scientists who are trained in biochemistry. They study chemical processes and chemical transformations in living organisms. Biochemists study DNA, proteins and Cell (biology), cell parts. The word "biochemist" is a portmanteau of ...
, biophysicists, chemists
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
and physicists
A physicist is a scientist who specializes in the field of physics, which encompasses the interactions of matter and energy at all length and time scales in the physical universe.
Physicists generally are interested in the root or ultimate caus ...
have largely adopted this definition, leading to some conflict in the biological community over an issue of language.
Proteins forming amyloids in diseases
To date, 37 human proteins have been found to form amyloid inpathology
Pathology is the study of the causes and effects of disease or injury. The word ''pathology'' also refers to the study of disease in general, incorporating a wide range of biology research fields and medical practices. However, when used in ...
and be associated with well-defined diseases. The International Society of Amyloidosis classifies amyloid fibrils and their associated diseases based upon associated proteins (for example ATTR is the group of diseases and associated fibrils formed by TTR). A table is included below.
Non-disease and functional amyloids
Many examples of non-pathological amyloid with a well-defined physiological role have been identified in various organisms, includinghuman
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, cultu ...
. These may be termed as functional or physiological or native amyloid.
* Functional amyloid in Homo sapiens
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, culture, ...
:
** Intralumenal domain of melanocyte protein PMEL
** Peptide/protein hormones stored as amyloids within endocrine secretory granules
** Receptor-interacting serine/threonine-protein kinase 1/3 ( RIP1/ RIP3)
** Fragments of prostatic acid phosphatase and semenogelins
* Functional amyloid in other organisms:
** Curli fibrils produced by '' E. coli,'' '' Salmonella, ''and a few other members of the Enterobacteriales
Enterobacterales is an order of Gram-negative, non-spore forming, facultatively anaerobic, rod-shaped bacteria with the class Gammaproteobacteria. The type genus of this order is ''Enterobacter.''
The name Enterobacterales is derived from the ...
(Csg). The genetic elements (operons
In genetics, an operon is a functioning unit of DNA containing a cluster of genes under the control of a single promoter. The genes are transcribed together into an mRNA strand and either translated together in the cytoplasm, or undergo splic ...
) encoding the curli system are phylogenetic widespread and can be found in at least four bacterial phyla. This suggest that many more bacteria may express curli fibrils.
** GvpA, forming the walls of particular Gas vesicle
Gas vesicles, also known as gas vacuoles, are nanocompartments in certain prokaryotic organisms, which help in buoyancy. Gas vesicles are composed entirely of protein; no lipids or carbohydrates have been detected.
Function
Gas vesicles occur ...
s, i.e. the buoyancy organelles of aquatic archaea and eubacteria
** Fap fibrils in various species of ''Pseudomonas
''Pseudomonas'' is a genus of Gram-negative, Gammaproteobacteria, belonging to the family Pseudomonadaceae and containing 191 described species. The members of the genus demonstrate a great deal of metabolic diversity and consequently are able t ...
''
** Chaplins from ''Streptomyces coelicolor
''Streptomyces albidoflavus'' is a bacterium species from the genus of ''Streptomyces'' which has been isolated from soil from Poland. ''Streptomyces albidoflavus'' produces dibutyl phthalate and streptothricins.
Small noncoding RNA
Bacter ...
''
** Spidroin
Spidroins are the main proteins in spider silk. Different types of spider silk contain different spidroins, all of which are members of a single protein family. The most-researched type of spidroins are the major ampullate silk proteins (MaS ...
from '' Trichonephila edulis'' (spider
Spiders ( order Araneae) are air-breathing arthropods that have eight legs, chelicerae with fangs generally able to inject venom, and spinnerets that extrude silk. They are the largest order of arachnids and rank seventh in total species ...
) (Spider silk
Spider silk is a protein fibre spun by spiders. Spiders use their silk to make webs or other structures, which function as sticky nets to catch other animals, or as nests or cocoons to protect their offspring, or to wrap up prey. They can ...
)
** Hydrophobins from Neurospora crassa
''Neurospora crassa'' is a type of red bread mold of the phylum Ascomycota. The genus name, meaning "nerve spore" in Greek, refers to the characteristic striations on the spores. The first published account of this fungus was from an infestation ...
and other fungi
** Fungal cell adhesion proteins forming cell surface amyloid regions with greatly increased binding strength
** Environmental biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular ...
s according to staining with amyloid specific dyes and antibodies.
** Tubular sheaths encasing Methanosaeta thermophila filaments
* Functional amyloid acting as prions
** Several yeast prions are based on an infectious amyloid, e.g. SI+(Sup35p
Sup35p is the ''Saccharomyces cerevisiae'' (a yeast) eukaryotic translation release factor. More specifically, it is the yeast eukaryotic release factor 3 (eRF3), which forms the translation termination complex with eRF1 ( Sup45p in yeast). This c ...
); RE3( Ure2p); IN+
IN, In or in may refer to:
Places
* India (country code IN)
* Indiana, United States (postal code IN)
* Ingolstadt, Germany (license plate code IN)
* In, Russia, a town in the Jewish Autonomous Oblast
Businesses and organizations
* Indepen ...
or NQ+(Rnq1p); WI1+(Swi1p) and CT8+(Cyc8p)
** Prion HET-s from '' Podospora anserina''
** Neuron-specific isoform of CPEB from '' Aplysia californica'' (marine snail)
Structure
 Amyloids are formed of long unbranched fibers that are characterized by an extended beta-sheet secondary structure in which individual
Amyloids are formed of long unbranched fibers that are characterized by an extended beta-sheet secondary structure in which individual beta strand
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
s (β-strands) (coloured arrows in the adjacent figure) are arranged in an orientation perpendicular to the long axis of the fiber. Such a structure is known as cross-β structure. Each individual fiber may be 7–13 nanometres in width and a few micrometre
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American spelling), also commonly known as a micron, is a unit of length in the International System of Unit ...
s in length. The main hallmarks recognised by different disciplines to classify protein aggregates as amyloid is the presence of a fibrillar morphology with the expected diameter, detected using transmission electron microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a g ...
(TEM) or atomic force microscopy (AFM), the presence of a cross-β secondary structure, determined with circular dichroism, FTIR, solid-state nuclear magnetic resonance
Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic pa ...
(ssNMR), X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
, or X-ray fiber diffraction (often considered the "gold-standard" test to see whether a structure contains cross-β fibres), and an ability to stain with specific dyes, such as Congo red
Congo red is an organic compound, the sodium salt of 3,3′-( ,1′-biphenyl4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid). It is an azo dye. Congo red is water-soluble, yielding a red colloidal solution; its solubility is greater in organic ...
, thioflavin T or thioflavin S.
The term "cross-β" was based on the observation of two sets of diffraction lines, one longitudinal and one transverse, that form a characteristic "cross" pattern. There are two characteristic scattering diffraction signals produced at 4.7 and 10 Ångstroms (0.47 nm and 1.0 nm), corresponding to the interstrand and stacking distances in beta sheets. The "stacks" of beta sheet are short and traverse the breadth of the amyloid fibril; the length of the amyloid fibril is built by aligned β-strands. The cross-β pattern is considered a diagnostic hallmark of amyloid structure.
Amyloid fibrils are generally composed of 1–8 protofilaments (one protofilament also corresponding to a fibril is shown in the figure), each 2–7 nm in diameter, that interact laterally as flat ribbons that maintain the height of 2–7 nm (that of a single protofilament) and are up to 30 nm wide; more often protofilaments twist around each other to form the typically 7–13 nm wide fibrils. Each protofilament possesses the typical cross-β structure and may be formed by 1–6 β-sheets (six are shown in the figure) stacked on each other. Each individual protein molecule can contribute one to several β-strands in each protofilament and the strands can be arranged in antiparallel β-sheets, but more often in parallel β-sheets. Only a fraction of the polypeptide chain is in a β-strand conformation in the fibrils, the remainder forms structured or unstructured loops or tails.
For a long time our knowledge of the atomic-level structure of amyloid fibrils was limited by the fact that they are unsuitable for the most traditional methods for studying protein structures. Recent years have seen progress in experimental methods, including solid-state NMR
Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic pa ...
spectroscopy and Cryo-Electron Microscopy
Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample s ...
. Combined, these methods have provided 3D atomic structures of amyloid fibrils formed by amyloid β peptides, α-synuclein, tau, and the FUS protein, associated with various neurodegenerative diseases.
X-ray diffraction studies of microcrystals revealed atomistic details of core region of amyloid, although only for simplified peptides having a length remarkably shorter than that of peptides or proteins involved in disease. The crystallographic structures show that short stretches from amyloid-prone regions of amyloidogenic proteins run perpendicular to the filament axis, consistent with the "cross-β" feature of amyloid structure. They also reveal a number of characteristics of amyloid structures – neighboring β-sheets are tightly packed together via an interface devoid of water (therefore referred to as dry interface), with the opposing β-strands slightly offset from each other such that their side-chains interdigitate. This compact dehydrated interface created was termed a steric-zipper interface. There are eight theoretical classes of steric-zipper interfaces, dictated by the directionality of the β-sheets (parallel and anti-parallel) and symmetry between adjacent β-sheets. A limitation of X-ray crystallography for solving amyloid structure is represented by the need to form microcrystals, which can be achieved only with peptides shorter than those associated with disease.
Although bona fide amyloid structures always are based on intermolecular β-sheets, different types of "higher order" tertiary folds have been observed or proposed. The β-sheets may form a β-sandwich, or a β-solenoid which may be either β-helix or β-roll. Native-like amyloid fibrils in which native β-sheet containing proteins maintain their native-like structure in the fibrils have also been proposed.
One complicating factor in studies of amyloidogenic polypeptides is that identical polypeptides can fold into multiple distinct amyloid conformations. This phenomenon is typically described as ''amyloid polymorphism''.
It has notable biological consequences given that it is thought to explain the prion strain phenomenon.
Formation
Amyloid is formed through thepolymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
of hundreds to thousands of monomeric peptides or proteins into long fibers. Amyloid formation involves a '' lag phase'' (also called '' nucleation phase''), an ''exponential
Exponential may refer to any of several mathematical topics related to exponentiation, including:
*Exponential function, also:
**Matrix exponential, the matrix analogue to the above
*Exponential decay, decrease at a rate proportional to value
*Expo ...
phase'' (also called ''growth phase'') and a ''plateau
In geology and physical geography, a plateau (; ; ), also called a high plain or a tableland, is an area of a highland consisting of flat terrain that is raised sharply above the surrounding area on at least one side. Often one or more sides ...
phase'' (also called ''saturation phase''), as shown in the figure. Indeed, when the quantity of fibrils is plotted versus time, a sigmoidal time course is observed reflecting the three distinct phases.
In the simplest model of 'nucleated polymerization' (marked by red arrows in the figure below), individual unfolded or partially unfolded polypeptide chains
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
(monomers) convert into a nucleus (monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
or oligomer) via a thermodynamically unfavourable process that occurs early in the lag phase. Fibrils grow subsequently from these nuclei through the addition of monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s in the exponential phase.
A different model, called 'nucleated conformational conversion' and marked by blue arrows in the figure below, was introduced later on to fit some experimental observations: monomers have often been found to convert rapidly into misfolded and highly disorganized oligomers distinct from nuclei. Only later on, will these aggregates reorganise structurally into nuclei, on which other disorganised oligomers will add and reorganise through a templating or induced-fit mechanism (this 'nucleated conformational conversion' model), eventually forming fibrils.
Normally folded proteins have to unfold partially before aggregation can take place through one of these mechanisms. In some cases, however, folded proteins can aggregate without crossing the major energy barrier for unfolding, by populating native-like conformations as a consequence of thermal fluctuations
In statistical mechanics, thermal fluctuations are random deviations of a system from its average state, that occur in a system at equilibrium.In statistical mechanics they are often simply referred to as fluctuations. All thermal fluctuations b ...
, ligand release or local unfolding occurring in particular circumstances. In these native-like conformations, segments that are normally buried or structured in the fully folded and possessing a high propensity to aggregate become exposed to the solvent or flexible, allowing the formation of native-like aggregates, which convert subsequently into nuclei and fibrils. This process is called 'native-like aggregation' (green arrows in the figure) and is similar to the 'nucleated conformational conversion' model.
A more recent, modern and thorough model of amyloid fibril formation involves the intervention of secondary events, such as 'fragmentation', in which a fibril breaks into two or more shorter fibrils, and 'secondary nucleation', in which fibril surfaces (not fibril ends) catalyze the formation of new nuclei. Both secondary events increase the number of fibril ends able to recruit new monomers or oligomers, therefore accelerating fibril formation through a positive feedback mechanism. These events add to the well recognised steps of primary nucleation (formation of the nucleus from the monomers through one of models described above), fibril elongation (addition of monomers or oligomers to growing fibril ends) and dissociation (opposite process).
Such a new model is described in the figure on the right and involves the utilization of a master equation
In physics, chemistry and related fields, master equations are used to describe the time evolution of a system that can be modelled as being in a probabilistic combination of states at any given time and the switching between states is determined ...
that includes all steps of amyloid fibril formation, i.e. primary nucleation, fibril elongation, secondary nucleation and fibril fragmentation. The rate constants of the various steps can be determined from a global fit of a number of time courses of aggregation (for example ThT fluorescence emission versus time) recorded at different protein concentrations. The general master equation approach to amyloid fibril formation with secondary pathways has been developed by Knowles, Vendruscolo, CohenMichaels
and coworkers and considers the time evolution of the concentration of fibrils of length (here represents the number of monomers in an aggregate). where denotes the
Kronecker delta
In mathematics, the Kronecker delta (named after Leopold Kronecker) is a function of two variables, usually just non-negative integers. The function is 1 if the variables are equal, and 0 otherwise:
\delta_ = \begin
0 &\text i \neq j, \\
1 & ...
. The physical interpretation of the various terms in the above master equation is straight forward: the terms on the first line describe the growth of fibrils via monomer addition with rate constant (elongation). The terms on the second line describe monomer dissociation, i.e. the inverse process of elongation. is the rate constant of monomer dissociation. The terms on the third line describe the effect of fragmentation, which is assumed to occur homogeneously along fibrils with rate constant . Finally, the terms on the last line describe primary and secondary nucleation respectively. Note that the rate of secondary nucleation is proportional to the mass of aggregates, defined as .
Following this analytical approach, it has become apparent that the lag phase does not correspond necessarily to only nucleus formation, but rather results from a combination of various steps. Similarly, the exponential phase is not only fibril elongation, but results from a combination of various steps, involving primary nucleation, fibril elongation, but also secondary events. A significant quantity of fibrils resulting from primary nucleation and fibril elongation may be formed during the lag phase and secondary steps, rather than only fibril elongation, can be the dominant processes contributing to fibril growth during the exponential phase. With this new model, any perturbing agents of amyloid fibril formation, such as putative drugs, metabolites
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, ...
, mutations, chaperones, etc., can be assigned to a specific step of fibril formation.
Amino acid sequence and amyloid formation
In general, amyloidpolymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ization (aggregation or non-covalent polymerization) is sequence-sensitive, that is mutations in the sequence can induce or prevent self-assembly. For example, humans produce amylin
Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by sl ...
, an amyloidogenic peptide associated with type II diabetes, but in rats and mice prolines are substituted in critical locations and amyloidogenesis does not occur. Studies comparing synthetic to recombinant β amyloid peptide in assays measuring rate of fibrillation, fibril homogeneity, and cellular toxicity showed that recombinant β amyloid peptide has a faster fibrillation rate and greater toxicity than synthetic β amyloid peptide.
There are multiple classes of amyloid-forming polypeptide sequences. Glutamine-rich polypeptides are important in the amyloidogenesis of Yeast and mammalian prions
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It i ...
, as well as trinucleotide repeat disorders including Huntington's disease
Huntington's disease (HD), also known as Huntington's chorea, is a neurodegenerative disease that is mostly inherited. The earliest symptoms are often subtle problems with mood or mental abilities. A general lack of coordination and an uns ...
. When glutamine-rich polypeptides are in a β-sheet conformation, glutamines can brace the structure by forming inter-strand hydrogen bonding between its amide carbonyls and nitrogens of both the backbone and side chains. The onset age for Huntington's disease shows an inverse correlation with the length of the polyglutamine sequence, with analogous findings in a '' C. elegans'' model system with engineered polyglutamine peptides.
Other polypeptides and proteins such as amylin
Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by sl ...
and the β amyloid peptide do not have a simple consensus sequence and are thought to aggregate through the sequence segments enriched with hydrophobic residues, or residues with high propensity to form β-sheet structure. Among the hydrophobic residues, aromatic amino-acids are found to have the highest amyloidogenic propensity.
Cross-polymerization (fibrils of one polypeptide sequence causing other fibrils of another sequence to form) is observed in vitro and possibly in vivo. This phenomenon is important, since it would explain interspecies prion propagation and differential rates of prion propagation, as well as a statistical link between Alzheimer's and type 2 diabetes. In general, the more similar the peptide sequence the more efficient cross-polymerization is, though entirely dissimilar sequences can cross-polymerize and highly similar sequences can even be "blockers" that prevent polymerization.
Amyloid toxicity
The reasons why amyloid cause diseases are unclear. In some cases, the deposits physically disrupt tissue architecture, suggesting disruption of function by some bulk process. An emerging consensus implicates prefibrillar intermediates, rather than mature amyloid fibers, in causing cell death, particularly in neurodegenerative diseases. The fibrils are, however, far from innocuous, as they keep the protein homeostasis network engaged, release oligomers, cause the formation of toxic oligomers via secondary nucleation, grow indefinitely spreading from district to district and, in some cases, may be toxic themselves. Calcium dysregulation has been observed to occur early in cells exposed to protein oligomers. These small aggregates can form ion channels through lipid bilayer membranes and activate NMDA and AMPA receptors. Channel formation has been hypothesized to account for calcium dysregulation and mitochondrial dysfunction by allowing indiscriminate leakage of ions across cell membranes. Studies have shown that amyloid deposition is associated with mitochondrial dysfunction and a resulting generation ofreactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () p ...
(ROS), which can initiate a signalling pathway leading to apoptosis. There are reports that indicate amyloid polymers (such as those of huntingtin, associated with Huntington's disease) can induce the polymerization of essential amyloidogenic proteins, which should be deleterious to cells. Also, interaction partners of these essential proteins can also be sequestered.
All these mechanisms of toxicity are likely to play a role. In fact, the aggregation of a protein generates a variety of aggregates, all of which are likely to be toxic to some degree. A wide variety of biochemical, physiological and cytological perturbations has been identified following the exposure of cells and animals to such species, independently of their identity. The oligomers have also been reported to interact with a variety of molecular targets. Hence, it is unlikely that there is a unique mechanism of toxicity or a unique cascade of cellular events. The misfolded nature of protein aggregates causes a multitude of aberrant interactions with a multitude of cellular components, including membranes, protein receptors, soluble proteins, RNAs, small metabolites, etc.
Histological staining
In the clinical setting, amyloid diseases are typically identified by a change in the spectroscopic properties of planararomatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
dyes such as thioflavin T, congo red
Congo red is an organic compound, the sodium salt of 3,3′-( ,1′-biphenyl4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic acid). It is an azo dye. Congo red is water-soluble, yielding a red colloidal solution; its solubility is greater in organic ...
or NIAD-4. In general, this is attributed to the environmental change, as these dyes intercalate between beta-strands to confine their structure.
Congo Red positivity remains the gold standard for diagnosis of amyloidosis. In general, binding of Congo Red to amyloid plaques produces a typical apple-green birefringence when viewed under cross-polarized light. Recently, significant enhancement of fluorescence quantum yield of NIAD-4 was exploited to super-resolution
Super-resolution imaging (SR) is a class of techniques that enhance (increase) the resolution of an imaging system. In optical SR the diffraction limit of systems is transcended, while in geometrical SR the resolution of digital imaging sensors ...
fluorescence imaging of amyloid fibrils and oligomers. To avoid nonspecific staining, other histology
Histology,
also known as microscopic anatomy or microanatomy, is the branch of biology which studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures vi ...
stains, such as the hematoxylin and eosin stain, are used to quench the dyes' activity in other places such as the nucleus, where the dye might bind. Modern antibody technology and immunohistochemistry has made specific staining easier, but often this can cause trouble because epitopes can be concealed in the amyloid fold; in general, an amyloid protein structure is a different conformation from the one that the antibody recognizes.
See also
* JUNQ and IPOD *Proteopathy
In medicine, proteinopathy (; 'pref''. protein -pathy 'suff''. disease proteinopathies ''pl''.; proteinopathic ''adj''), or proteopathy, protein conformational disorder, or protein misfolding disease refers to a class of diseases in which certa ...
* Protein aggregation predictorsReferences
External links
Bacterial Inclusion Bodies Contain Amyloid-Like Structure
at SciVee
Amyloid Cascade Hypothesis
Amyloid: Journal of Protein Folding Disorders web page
{{Amyloidosis Amyloidosis Histopathology Structural proteins