aminoallyl nucleotide on:
[Wikipedia]
[Google]
[Amazon]
 Aminoallyl nucleotide is a nucleotide with a modified base containing an
Aminoallyl nucleotide is a nucleotide with a modified base containing an
 The goal of combining fluorescence and nucleic acids has been to provide a non- isotopic tag that is detectable to study DNA or
The goal of combining fluorescence and nucleic acids has been to provide a non- isotopic tag that is detectable to study DNA or
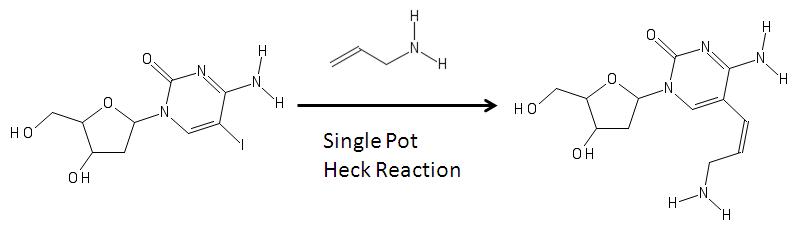 In the image above, on the left is a modified nucleoside with an
In the image above, on the left is a modified nucleoside with an
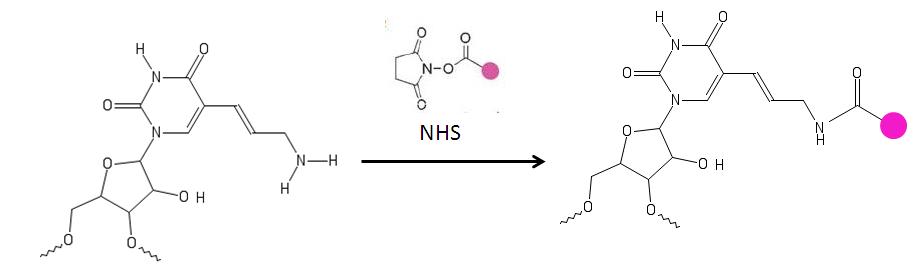
 Another process which uses aminoallyl labeling is NASBA ( Nucleic Acid Sequence Based Amplification), a highly sensitive technique for amplifying RNA. In this specific case, the aaUTP modified RNAs were tagged with fluorescent market Cy3. NASBA combined with aminoallyl-UTP labeling is very useful for many different areas of microbial diagnostics including environmental monitoring, bio threat detection, industrial process monitoring and clinical microbiology. DNA microarray is another method which utilizes specifically AA-NTP's making DNA microarray testing quicker and cheaply.
Post-synthesis labeling avoids the problems found in direct enzymatic incorporation of Cy-labeled dNTPs by generating probes with equal labeling effectiveness. With indirect labeling, amine-modified NTPs are incorporated during
Another process which uses aminoallyl labeling is NASBA ( Nucleic Acid Sequence Based Amplification), a highly sensitive technique for amplifying RNA. In this specific case, the aaUTP modified RNAs were tagged with fluorescent market Cy3. NASBA combined with aminoallyl-UTP labeling is very useful for many different areas of microbial diagnostics including environmental monitoring, bio threat detection, industrial process monitoring and clinical microbiology. DNA microarray is another method which utilizes specifically AA-NTP's making DNA microarray testing quicker and cheaply.
Post-synthesis labeling avoids the problems found in direct enzymatic incorporation of Cy-labeled dNTPs by generating probes with equal labeling effectiveness. With indirect labeling, amine-modified NTPs are incorporated during
Example protocol
by Holly Bennet and Joe DeRisi originated at Rosetta Informatics modified by Chris Seidel.{{cite web, last=Seidel, first=Chris, title=Fluorescent Probe Preparation, url=http://www.pangloss.com/seidel/Protocols/amino-allylRT.html, access-date=24 March 2014 Nucleic acids Nucleotides Molecular biology Biotechnology Synthetic biology
allylamine
Allylamine is an organic compound with the formula C3H5NH2. This colorless liquid is the simplest stable unsaturated amine.
Production and reactions
All three allylamines, mono-, di-, and triallylamine, are produced by the treating allyl chlor ...
. They are used in post-labeling of nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s by fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
detection in microarray
A microarray is a multiplex lab-on-a-chip. Its purpose is to simultaneously detect the expression of thousands of genes from a sample (e.g. from a tissue). It is a two-dimensional array on a solid substrate—usually a glass slide or silicon t ...
. They are reactive with N-Hydroxysuccinimide ester group which helps attach a fluorescent dye
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
to the primary amino group on the nucleotide. These nucleotides are known as 5-(3-aminoallyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
)-nucleotides since the aminoallyl group is usually attached to carbon 5 of the pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
ring of uracil
Uracil () (symbol U or Ura) is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by ...
or cytosine
Cytosine () ( symbol C or Cyt) is one of the four nucleobases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an am ...
. The primary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
group in the aminoallyl moiety is aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
and thus more reactive compared to the amine groups that are directly attached to the rings (aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
) of the bases. Common names of aminoallyl nucleosides
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide ...
are initially abbreviated with aa- or AA- to indicate aminoallyl. The 5-carbon sugar is indicated with or without the lowercase "d" indicating deoxyribose
Deoxyribose, or more precisely 2-deoxyribose, is a monosaccharide with idealized formula H−(C=O)−(CH2)−(CHOH)3−H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of a hydroxy group. D ...
if included or ribose
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, , is a component of the ribonucleotides from which RNA is built, and so this compo ...
if not. Finally the nitrogenous base
Nucleobases, also known as ''nitrogenous bases'' or often simply ''bases'', are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic b ...
and number of phosphates
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
are indicated (i.e. aa-UTP = aminoallyl uridine triphosphate
Polyphosphates are salts or esters of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic ring structures. In biology, the polyphosphate e ...
).
History
 The goal of combining fluorescence and nucleic acids has been to provide a non- isotopic tag that is detectable to study DNA or
The goal of combining fluorescence and nucleic acids has been to provide a non- isotopic tag that is detectable to study DNA or RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
. This type of labeling allows scientists to study DNA or RNA in their structure, function, or formation with other nucleic acids. The first base modification for fluorescent labeling occurred in 1971 with a 4-thiouridine
4-Thiouridine is an atypical nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) ...
and 4-thiouracil
4-Thiouracil is a heterocyclic organic compound having a pyrimidine skeleton. It is a derivative of the nucleobase uracil with a sulfur instead of oxygen in position 4. It is found naturally in the 4-thiouridine
4-Thiouridine is an atypical nucle ...
. This research along with others, which included various types of direct and non-direct labeling via: analogs, addition via enzymes, or other methods made labeling of nucleotides much safer for scientist to study DNA.
As instrumentation and technologies become more advanced in the field of DNA microarray
A DNA microarray (also commonly known as DNA chip or biochip) is a collection of microscopic DNA spots attached to a solid surface. Scientists use DNA microarrays to measure the expression levels of large numbers of genes simultaneously or to ...
, better reagents and techniques will be needed to further scientific studies. Fluorescent labeling with Cy3 was shown to be more insufficient and skew results; the method of aminoallyl nucleotide incorporation was opted instead. Using aminoallyl nucleotides as indirect fluorescent labeling seemed to nullify the sensitivity issues seen in cyanine-labeling.
Synthesis
Aminoallylnucleosides
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide ...
can be synthesized via Heck coupling
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
as shown in the image below.
 In the image above, on the left is a modified nucleoside with an
In the image above, on the left is a modified nucleoside with an iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
(the iodine is added via electrophilic halogenation
In organic chemistry, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution. This organic reaction is typical of aromatic compounds and a very useful method for adding substituents to an aromatic system.
:
A few ...
) in the fifth carbon in the pyrimidine ring. Its formation can be associated with a reaction with an allylamine and various reagents via heck coupling are able to remove the halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
group from the base and add the allylamine to become the aminoallyl nucleoside shown on the right. The product on the right is then used to in molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physi ...
in RNA synthesis.
Other reactions include using a single pot synthesis with other halogens
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
.
Reaction
The primary amine on the aminoallyl nucleotide reacts with amino-reactive dyes such as acyanine
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic s ...
and patented dyes which contain a reactive leaving group, such as a succinimidyl ester (NHS
The National Health Service (NHS) is the umbrella term for the publicly funded healthcare systems of the United Kingdom (UK). Since 1948, they have been funded out of general taxation. There are three systems which are referred to using the " ...
).The amine groups directly attached to the ring of the base are not affected. These nucleotides are used for labeling DNA.

Uses
Aminoallyl NTPs are used for indirect DNA labeling in PCR,nick translation
Nick translation (or head translation), developed in 1977 by Peter Rigby and Paul Berg, is a tagging technique in molecular biology in which DNA Polymerase I is used to replace some of the nucleotides of a DNA sequence with their labeled analogu ...
, primer extension
Primer extension is a technique whereby the 5' ends of RNA can be mapped - that is, they can be sequenced and properly identified.
Primer extension can be used to determine the start site of transcription (the end site cannot be determined by th ...
s and cDNA
In genetics, complementary DNA (cDNA) is DNA synthesized from a single-stranded RNA (e.g., messenger RNA (mRNA) or microRNA (miRNA)) template in a reaction catalyzed by the enzyme reverse transcriptase. cDNA is often used to express a speci ...
synthesis. These labeled NTPs are helpful because of their application in molecular biology labs where they do not have the capacity to handle radioactive material. For example, 5-(3-Aminoallyl)-Uridine(AA-UTPs) are more effective for high density labeling of DNA than pre-labeling the DNA. After the enzymatic addition of the NTPs, amine reactant fluorescent dyes can be added for detection of the DNA molecule. When incorporated into DNA or RNA molecules by DNA/RNA polymerase
A polymerase is an enzyme ( EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by copying a DNA template strand using base- ...
, 5-(3-aminoallyl)-UTP provide a reactive group for the addition of other chemical groups. Thus aminoallyl modified DNA or RNA can be labeled with any compound which has an amine-reactive group.
aa-NTPs incorporated into DNA/RNA in combination with a secondary dye coupling reagents can probe for an array analysis.
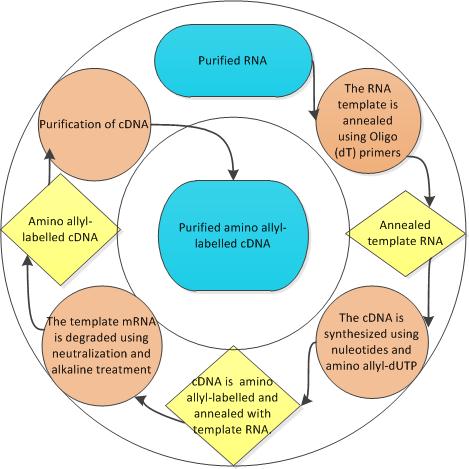
cDNA relies on aminoallyl labeling for detection purposes. Although direct labeling of dNTP is the quickest and cheapest method of fluorescent labeling, it is disadvantageous as the sequence allows for only one modified nucleotide for use. Another disadvantage of direct labeling is the bulky nucleotides, however this can be overcome by indirect labeling using aminoallyl modified nucleotides. An easy way to check for labeling success is the color;Good labeling will result in visible blue (Cy5) or red (Cy3) color in the final material.
 Another process which uses aminoallyl labeling is NASBA ( Nucleic Acid Sequence Based Amplification), a highly sensitive technique for amplifying RNA. In this specific case, the aaUTP modified RNAs were tagged with fluorescent market Cy3. NASBA combined with aminoallyl-UTP labeling is very useful for many different areas of microbial diagnostics including environmental monitoring, bio threat detection, industrial process monitoring and clinical microbiology. DNA microarray is another method which utilizes specifically AA-NTP's making DNA microarray testing quicker and cheaply.
Post-synthesis labeling avoids the problems found in direct enzymatic incorporation of Cy-labeled dNTPs by generating probes with equal labeling effectiveness. With indirect labeling, amine-modified NTPs are incorporated during
Another process which uses aminoallyl labeling is NASBA ( Nucleic Acid Sequence Based Amplification), a highly sensitive technique for amplifying RNA. In this specific case, the aaUTP modified RNAs were tagged with fluorescent market Cy3. NASBA combined with aminoallyl-UTP labeling is very useful for many different areas of microbial diagnostics including environmental monitoring, bio threat detection, industrial process monitoring and clinical microbiology. DNA microarray is another method which utilizes specifically AA-NTP's making DNA microarray testing quicker and cheaply.
Post-synthesis labeling avoids the problems found in direct enzymatic incorporation of Cy-labeled dNTPs by generating probes with equal labeling effectiveness. With indirect labeling, amine-modified NTPs are incorporated during reverse transcription
A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, ...
, RNA amplification, or PCR. Amino allyl-NTPs are incorporated with similar efficiency as unmodified NTPs during polymerization.
Concerns with labeling:
The amine group, in aminoallyl-modified nucleotide, is reactive with dyes such as the cyanine series, or other patented dyes. A problem arises when the dyes react with buffering agents which are necessary for the proper storage of the nucleotides. However, a carbonate buffer can be used to overcome this problem.
See also
* NASBA * PCR *Nick Translation
Nick translation (or head translation), developed in 1977 by Peter Rigby and Paul Berg, is a tagging technique in molecular biology in which DNA Polymerase I is used to replace some of the nucleotides of a DNA sequence with their labeled analogu ...
* cDNA
In genetics, complementary DNA (cDNA) is DNA synthesized from a single-stranded RNA (e.g., messenger RNA (mRNA) or microRNA (miRNA)) template in a reaction catalyzed by the enzyme reverse transcriptase. cDNA is often used to express a speci ...
* Microarray
A microarray is a multiplex lab-on-a-chip. Its purpose is to simultaneously detect the expression of thousands of genes from a sample (e.g. from a tissue). It is a two-dimensional array on a solid substrate—usually a glass slide or silicon t ...
* Fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with se ...
*Reverse Transcription
A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, ...
References
External links
Example protocol
by Holly Bennet and Joe DeRisi originated at Rosetta Informatics modified by Chris Seidel.{{cite web, last=Seidel, first=Chris, title=Fluorescent Probe Preparation, url=http://www.pangloss.com/seidel/Protocols/amino-allylRT.html, access-date=24 March 2014 Nucleic acids Nucleotides Molecular biology Biotechnology Synthetic biology