Acetylene on:
[Wikipedia]
[Google]
[Amazon]
Acetylene ( systematic name: ethyne) is the
Material Safety and Data Sheet – Acetylene
As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a
 Until the 1950s, when oil supplanted
Until the 1950s, when oil supplanted CaC2 + 2H2O -> Ca(OH)2 + C2H_
Calcium carbide production requires extremely high temperatures, ~2000 °C, necessitating the use of an electric arc furnace. In the US, this process was an important part of the late-19th century revolution in chemistry enabled by the massive hydroelectric power project at

C2H2 + H2O -> CH3CHO
Acetylene is a moderately common chemical in the universe, often associated with the atmospheres of gas giants. One curious discovery of acetylene is on Enceladus, a moon of Saturn. Natural acetylene is believed to form from catalytic decomposition of long-chain hydrocarbons at temperatures of and above. Since such temperatures are highly unlikely on such a small distant body, this discovery is potentially suggestive of catalytic reactions within that moon, making it a promising site to search for prebiotic chemistry.
 The hydration of acetylene is a vinylation reaction, but the resulting vinyl alcohol isomerizes to
The hydration of acetylene is a vinylation reaction, but the resulting vinyl alcohol isomerizes to
 The reaction with
The reaction with
 :
: With
With
 :
: :
:Fe(CO)5 + 4 C2H2 + 2 H2O -> 2 C6H4(OH)2 + FeCO3 at basic conditions (50–, 20–).
In the presence of certain transition metals, alkynes undergo alkyne metathesis.
Metal acetylides, species of the formula , are also common. Copper(I) acetylide and silver acetylide can be formed in aqueous solutions with ease due to a poor
HC#CH + RM -> RH + HC#CM
Various organometallic and inorganic reagents are effective.
Acetylene Production Plant and Detailed Process
Acetylene at Chemistry Comes Alive!
*
Movie explaining acetylene formation from calcium carbide and the explosive limits forming fire hazards
at '' The Periodic Table of Videos'' (University of Nottingham)
CDC – NIOSH Pocket Guide to Chemical Hazards – Acetylene
{{Authority control Alkynes Fuel gas Industrial gases Synthetic fuel technologies Explosive chemicals Explosive gases
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula and structure . It is a hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
.Compressed Gas Association (1995Material Safety and Data Sheet – Acetylene
As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a
triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°.
Discovery
Acetylene was discovered in 1836 by Edmund Davy, who identified it as a "new carburet of hydrogen". It was an accidental discovery while attempting to isolatepotassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmos ...
metal. By heating potassium carbonate with carbon at very high temperatures, he produced a residue of what is now known as potassium carbide, (K2C2), which reacted with water to release the new gas. It was rediscovered in 1860 by French chemist Marcellin Berthelot, who coined the name ''acétylène''. Berthelot's empirical formula for acetylene (C4H2), as well as the alternative name "''quadricarbure d'hydrogène''" (hydrogen quadricarbide), were incorrect because many chemists at that time used the wrong atomic mass for carbon (6 instead of 12). Berthelot was able to prepare this gas by passing vapours of organic compounds (methanol, ethanol, etc.) through a red hot tube and collecting the effluent. He also found that acetylene was formed by sparking electricity through mixed cyanogen
Cyanogen is the chemical compound with the formula ( C N)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molec ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
gases. Berthelot later obtained acetylene directly by passing hydrogen between the poles of a carbon arc.
Preparation
Since the 1950s, acetylene has mainly been manufactured by the partialcombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combus ...
of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane ...
. It is a recovered side product in production of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
by cracking of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s. Approximately 400,000 tonnes were produced by this method in 1983. Its presence in ethylene is usually undesirable because of its explosive character and its ability to poison Ziegler–Natta catalysts. It is selectively hydrogenated into ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
, usually using Pd– Ag catalysts.
 Until the 1950s, when oil supplanted
Until the 1950s, when oil supplanted coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when ...
as the chief source of reduced carbon, acetylene (and the aromatic fraction from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat pso ...
) was the main source of organic chemicals in the chemical industry. It was prepared by the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysi ...
of calcium carbide, a reaction discovered by Friedrich Wöhler
Friedrich Wöhler () FRS(For) Hon FRSE (31 July 180023 September 1882) was a German chemist known for his work in inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the fi ...
in 1862 and still familiar to students:
: Niagara Falls
Niagara Falls () is a group of three waterfalls at the southern end of Niagara Gorge, spanning the Canada–United States border, border between the Provinces and territories of Canada, province of Ontario in Canada and the U.S. state, state ...
.
Bonding
In terms of valence bond theory, in each carbon atom the 2s orbital hybridizes with one 2p orbital thus forming an sp hybrid. The other two 2p orbitals remain unhybridized. The two ends of the two sp hybrid orbital overlap to form a strong σ valence bond between the carbons, while on each of the other two ends hydrogen atoms attach also by σ bonds. The two unchanged 2p orbitals form a pair of weaker π bonds. Since acetylene is a linear symmetrical molecule, it possesses the D∞h point group.Physical properties
Changes of state
At atmospheric pressure, acetylene cannot exist as a liquid and does not have a melting point. Thetriple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the ...
on the phase diagram corresponds to the melting point (−80.8 °C) at the minimal pressure at which liquid acetylene can exist (1.27 atm). At temperatures below the triple point, solid acetylene can change directly to the vapour (gas) by sublimation. The sublimation point at atmospheric pressure is −84.0 °C.
Other
At room temperature, the solubility of acetylene inacetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
is 27.9 g per kg. For the same amount of dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majo ...
(DMF), the solubility is 51 g. At
20.26 bar, the solubility increases to 689.0 and 628.0 g for acetone and DMF, respectively. These solvents are used in pressurized gas cylinders.
Applications
Welding
Approximately 20% of acetylene is supplied by the industrial gases industry for oxyacetylene gas welding and cutting due to the high temperature of the flame. Combustion of acetylene with oxygen produces a flame of over , releasing 11.8 kJ/g. Oxyacetylene is the hottest burning common fuel gas. Acetylene is the third-hottest natural chemical flame after dicyanoacetylene's andcyanogen
Cyanogen is the chemical compound with the formula ( C N)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molec ...
at . Oxy-acetylene welding was a popular welding process in previous decades. The development and advantages of arc-based welding processes have made oxy-fuel welding nearly extinct for many applications. Acetylene usage for welding has dropped significantly. On the other hand, oxy-acetylene welding ''equipment'' is quite versatile – not only because the torch is preferred for some sorts of iron or steel welding (as in certain artistic applications), but also because it lends itself easily to brazing, braze-welding, metal heating (for annealing or tempering, bending or forming), the loosening of corroded nuts and bolts, and other applications. Bell Canada
Bell Canada (commonly referred to as Bell) is a Canadian telecommunications company headquartered at 1 Carrefour Alexander-Graham-Bell in the borough of Verdun in Montreal, Quebec, Canada. It is an ILEC (incumbent local exchange carrier) in ...
cable-repair technicians still use portable acetylene-fuelled torch kits as a soldering
Soldering (; ) is a process in which two or more items are joined by melting and putting a filler metal ( solder) into the joint, the filler metal having a lower melting point than the adjoining metal. Unlike welding, soldering does not inv ...
tool for sealing lead sleeve splices in manholes and in some aerial locations. Oxyacetylene welding may also be used in areas where electricity is not readily accessible. Oxyacetylene cutting is used in many metal fabrication shops. For use in welding and cutting, the working pressures must be controlled by a regulator, since above , if subjected to a shockwave (caused, for example, by a flashback), acetylene decomposes explosively into hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
and carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
.

Portable lighting
Acetylene combustion produces a strong, bright light and the ubiquity of carbide lamps drove much acetylene commercialization in the early 20th century. Common applications included coastallighthouses
A lighthouse is a tower, building, or other type of physical structure designed to emit light from a system of lamps and lenses and to serve as a beacon for navigational aid, for maritime pilots at sea or on inland waterways.
Lighthouses mar ...
, street lights, and automobile
A car or automobile is a motor vehicle with wheels. Most definitions of ''cars'' say that they run primarily on roads, seat one to eight people, have four wheels, and mainly transport people instead of goods.
The year 1886 is regarded ...
and mining
Mining is the extraction of valuable minerals or other geological materials from the Earth, usually from an ore body, lode, vein, seam, reef, or placer deposit. The exploitation of these deposits for raw material is based on the econom ...
headlamps. In most of these applications, direct combustion is a fire hazard, and so acetylene has been replaced, first by incandescent lighting
An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated until it glows. The filament is enclosed in a glass bulb with a vacuum or inert gas to protect the filament from oxida ...
and many years later by low-power/high-lumen LEDs. Nevertheless, acetylene lamps remain in limited use in remote or otherwise inaccessible areas and in countries with a weak or unreliable central electric grid.
Plastics and acrylic acid derivatives
Acetylene can be semihydrogenated toethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
, providing a feedstock for a variety of polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including b ...
plastics. Another major application of acetylene, especially in China is its conversion to acrylic acid derivatives. These derivatives form products such as acrylic fibers, glass
Glass is a non- crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenchin ...
es, paints, resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on nat ...
s, and polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s.
Except in China, use of acetylene as a chemical feedstock has declined by 70% from 1965 to 2007 owing to cost and environmental considerations.
Niche applications
In 1881, the Russian chemist Mikhail Kucherov described thehydration Hydration may refer to:
* Hydrate, a substance that contains water
* Hydration enthalpy, energy released through hydrating a substance
* Hydration reaction, a chemical addition reaction where a hydroxyl group and proton are added to a compound
* ...
of acetylene to acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
using catalysts such as mercury(II) bromide. Before the advent of the Wacker process, this reaction was conducted on an industrial scale.
The polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many f ...
of acetylene with Ziegler–Natta catalysts produces polyacetylene films. Polyacetylene, a chain of CH centres with alternating single and double bonds, was one of the first discovered organic semiconductors. Its reaction with iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
produces a highly electrically conducting material. Although such materials are not useful, these discoveries led to the developments of organic semiconductors, as recognized by the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
in 2000 to Alan J. Heeger
Alan Jay Heeger (born January 22, 1936) is an American physicist, academic and Nobel Prize laureate in chemistry.
Heegar was elected as a member into the National Academy of Engineering in 2002 for co-founding the field of conducting polymers a ...
, Alan G MacDiarmid, and Hideki Shirakawa.
In the 1920s, pure acetylene was experimentally used as an inhalation anesthetic
An inhalational anesthetic is a chemical compound possessing general anesthetic properties that can be delivered via inhalation. They are administered through a face mask, laryngeal mask airway or tracheal tube connected to an anesthetic vapori ...
.
Acetylene is sometimes used for carburization
Carburising, carburizing (chiefly American English), or carburisation is a heat treatment process in which iron or steel absorbs carbon while the metal is heated in the presence of a carbon-bearing material, such as charcoal or carbon monoxide. ...
(that is, hardening) of steel when the object is too large to fit into a furnace.
Acetylene is used to volatilize carbon in radiocarbon dating
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon.
The method was de ...
. The carbonaceous material in an archeological sample is treated with lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense soli ...
metal in a small specialized research furnace to form lithium carbide
Lithium carbide, , often known as dilithium acetylide, is a chemical compound of lithium and carbon, an acetylide. It is an intermediate compound produced during radiocarbon dating procedures. is one of an extensive range of lithium-carbon compo ...
(also known as lithium acetylide). The carbide can then be reacted with water, as usual, to form acetylene gas to feed into a mass spectrometer to measure the isotopic ratio of carbon-14 to carbon-12.
Natural occurrence
The energy richness of the C≡C triple bond and the rather high solubility of acetylene in water make it a suitable substrate for bacteria, provided an adequate source is available. A number of bacteria living on acetylene have been identified. Theenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
acetylene hydratase Acetylene hydratase (, AH) is a bacterial enzyme, originally discovered in the anaerobic microorganism ''Pelobactor acetylenicus'', that catalyzes the non-redox hydration of acetylene to form acetaldehyde.
:C2H2 + H2O → CH3CHO
The mechanism is ...
catalyzes the hydration of acetylene to give acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
:
:Reactions
Vinylation reactions
In vinylation reactions, H−X compounds add across the triple bond.Alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
and phenols add to acetylene to give vinyl ethers. Thiols give vinyl thioethers. Similarly, vinylpyrrolidone and vinylcarbazole are produced industrially by vinylation of 2-pyrrolidone and carbazole.
: The hydration of acetylene is a vinylation reaction, but the resulting vinyl alcohol isomerizes to
The hydration of acetylene is a vinylation reaction, but the resulting vinyl alcohol isomerizes to acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
. The reaction is catalyzed by mercury salts. This reaction once was the dominant technology for acetaldehyde production, but it has been displaced by the Wacker process, which affords acetaldehyde by oxidation of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
, a cheaper feedstock. A similar situation applies to the conversion of acetylene to the valuable vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
by hydrochlorination vs the oxychlorination In organic chemistry, oxychlorination is a process for making C-Cl bonds. In contrast with direct use of Cl2, oxychlorination uses hydrogen chloride in combination with oxygen.{{Ullmann, author=M. Rossberg , display-authors=et al., title=Chlorinat ...
of ethylene.
Ethynylation
Acetylene adds to aldehydes andketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double b ...
to form α-ethynyl alcohols:
:formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
is used industrially in the production of butynediol, forming propargyl alcohol as the by-product. Copper acetylide is used as the catalyst.
Because halogens add across the triple bond, the substituted acetylenes difluoroacetylene, dichloroacetylene
Dichloroacetylene (DCA) is an organochlorine compound with the formula C2Cl2. It is a colorless, explosive liquid that has a sweet and "disagreeable" odor.
Production
Dichloroacetylene was first synthesized in 1930.
Ether solutions of dichloroa ...
, dibromoacetylene, and diiodoacetylene cannot be made directly from acetylene. A common workaround is to dehydrate vinyl dihaloethenols.
Carbonylation
Walter Reppe discovered that in the presence ofcatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s, acetylene reacts to give a wide range of industrially significant chemicals.
: :
: With
With carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
, acetylene reacts to give acrylic acid, or acrylic esters, which can be used to produce acrylic glass.
Organometallic chemistry
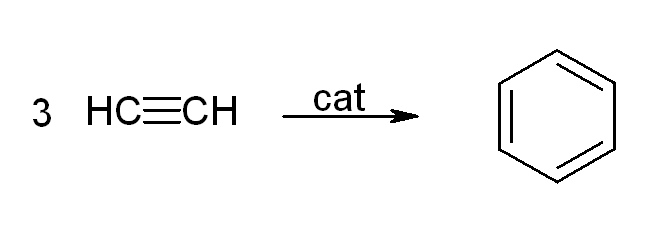
Acetylene and its derivatives (2-butyne, diphenylacetylene, etc.) form complexes with transition metals. Its bonding to the metal is somewhat similar to that of ethylene complexes. These complexes are intermediates in many catalytic reactions such asalkyne trimerisation
In organic chemistry, an alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applica ...
to benzene, tetramerization to cyclooctatetraene, and carbonylation to hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''pa ...
:
: :
: :
:solubility equilibrium Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reac ...
.
Acid-base reactions
Acetylene has a p''K''a of 25, acetylene can be deprotonated by a superbase to form an acetylide: :Safety and handling
Acetylene is not especially toxic, but when generated from calcium carbide, it can contain toxic impurities such as traces ofphosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
and arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications ...
, which give it a distinct garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plant in the genus '' Allium''. Its close relatives include the onion, shallot, leek, chive, Welsh onion and Chinese onion. It is native to South Asia, Central Asia and northeas ...
-like smell. It is also highly flammable, as are most light hydrocarbons, hence its use in welding. Its most singular hazard is associated with its intrinsic instability, especially when it is pressurized: under certain conditions acetylene can react in an exothermic addition-type reaction to form a number of products, typically benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
and/or vinylacetylene, possibly in addition to carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
. Consequently, acetylene, if initiated by intense heat or a shockwave, can decompose explosively if the absolute pressure of the gas exceeds about . Most regulators and pressure gauges on equipment report gauge pressure, and the safe limit for acetylene therefore is 101 kPagage, or 15 psig. It is therefore supplied and stored dissolved in acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
or dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majo ...
(DMF), contained in a gas cylinder
A gas cylinder is a pressure vessel for storage and containment of gases at above atmospheric pressure. High-pressure gas cylinders are also called ''bottles''. Inside the cylinder the stored contents may be in a state of compressed gas, vap ...
with a porous filling ( Agamassan), which renders it safe to transport and use, given proper handling. Acetylene cylinders should be used in the upright position to avoid withdrawing acetone during use.
Information on safe storage of acetylene in upright cylinders is provided by the OSHA, Compressed Gas Association, United States Mine Safety and Health Administration (MSHA), EIGA, and other agencies.
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
catalyses the decomposition of acetylene, and as a result acetylene should not be transported in copper pipes.
Cylinders should be stored in an area segregated from oxidizers to avoid exacerbated reaction in case of fire/leakage. Acetylene cylinders should not be stored in confined spaces, enclosed vehicles, garages, and buildings, to avoid unintended leakage leading to explosive atmosphere. In the US, National Electric Code (NEC) requires consideration for hazardous areas including those where acetylene may be released during accidents or leaks. Consideration may include electrical classification and use of listed Group A electrical components in USA. Further information on determining the areas requiring special consideration is in NFPA 497. In Europe, ATEX also requires consideration for hazardous areas where flammable gases may be released during accidents or leaks.
References
External links
Acetylene Production Plant and Detailed Process
Acetylene at Chemistry Comes Alive!
*
Movie explaining acetylene formation from calcium carbide and the explosive limits forming fire hazards
at '' The Periodic Table of Videos'' (University of Nottingham)
CDC – NIOSH Pocket Guide to Chemical Hazards – Acetylene
{{Authority control Alkynes Fuel gas Industrial gases Synthetic fuel technologies Explosive chemicals Explosive gases