Water Condensation on:
[Wikipedia]
[Google]
[Amazon]
(99.9839 °C)
, -
,
 Water vapor will only condense onto another surface when that surface is cooler than the dew point temperature, or when the water vapor equilibrium in air has been exceeded. When water vapor condenses onto a surface, a net warming occurs on that surface. The water molecule brings heat energy with it. In turn, the temperature of the atmosphere drops slightly. In the atmosphere, condensation produces clouds, fog and precipitation (usually only when facilitated by
Water vapor will only condense onto another surface when that surface is cooler than the dew point temperature, or when the water vapor equilibrium in air has been exceeded. When water vapor condenses onto a surface, a net warming occurs on that surface. The water molecule brings heat energy with it. In turn, the temperature of the atmosphere drops slightly. In the atmosphere, condensation produces clouds, fog and precipitation (usually only when facilitated by
 Water vapor and dry air density calculations at 0 °C:
* The molar mass of water is , as calculated from the sum of the atomic masses of its constituent
Water vapor and dry air density calculations at 0 °C:
* The molar mass of water is , as calculated from the sum of the atomic masses of its constituent
 Gaseous water represents a small but environmentally significant constituent of the
Gaseous water represents a small but environmentally significant constituent of the
/ref>
/ref> this has likely happened, possibly more than once. Scientists thus distinguish between non-condensable (driving) and condensable (driven) greenhouse gases, i.e., the above water vapor feedback.Lacis, A. et al., The role of long-lived greenhouse gases as principal LW control knob that governs the global surface temperature for past and future climate change, Tellus B, vol. 65 p. 19734, 2013
''Atmospheric Science: An Introductory Survey''
. Elsevier. Second Edition, 2006. . Page 8. and can be measured with a combination of land observations, weather balloons and satellites. The water content of the atmosphere as a whole is constantly depleted by precipitation. At the same time it is constantly replenished by evaporation, most prominently from oceans, lakes, rivers, and moist earth. Other sources of atmospheric water include combustion, respiration, volcanic eruptions, the transpiration of plants, and various other biological and geological processes. At any given time there is about 1.29 x 1016 litres (3.4 x 1015 gal.) of water in the atmosphere. The atmosphere holds 1 part in 2500 of the fresh water, and 1 part in 100,000 of the total water on Earth. The mean global content of water vapor in the atmosphere is roughly sufficient to cover the surface of the planet with a layer of liquid water about 25 mm deep. The mean annual precipitation for the planet is about 1 metre, a comparison which implies a rapid turnover of water in the air – on average, the residence time of a water molecule in the troposphere is about 9 to 10 days. Global mean water vapour is about 0.25% of the atmosphere by mass and also varies seasonally, in terms of contribution to atmospheric pressure between 2.62 hPa in July and 2.33 hPa in December. IPCC AR6 expresses medium confidence in increase of total water vapour at about 1-2% per decade; it is expected to increase by around 7% per °C of warming. Episodes of surface geothermal activity, such as volcanic eruptions and geysers, release variable amounts of water vapor into the atmosphere. Such eruptions may be large in human terms, and major explosive eruptions may inject exceptionally large masses of water exceptionally high into the atmosphere, but as a percentage of total atmospheric water, the role of such processes is trivial. The relative concentrations of the various gases emitted by volcanoes varies considerably according to the site and according to the particular event at any one site. However, water vapor is consistently the commonest volcanic gas; as a rule, it comprises more than 60% of total emissions during a subaerial eruption. Atmospheric water vapor content is expressed using various measures. These include vapor pressure, specific humidity, mixing ratio, dew point temperature, and relative humidity.

 Geological formations such as
Geological formations such as
National Science Digital Library – Water Vapor
Calculate the condensation of your exhaled breath
Water Vapor Myths: A Brief Tutorial
– PhyMetrix {{Authority control Greenhouse gases Atmospheric thermodynamics Forms of water Water in gas Psychrometrics Articles containing video clips
Boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
,
, -
, specific gas constant
, 461.5 J/( kg·K)
, -
, Heat of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
, 2.27 MJ/kg
, -
, Heat capacity
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidi ...
or boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Th ...
of liquid water or from the sublimation
Sublimation or sublimate may refer to:
* ''Sublimation'' (album), by Canvas Solaris, 2004
* Sublimation (phase transition), directly from the solid to the gas phase
* Sublimation (psychology), a mature type of defense mechanism
* Sublimate of mer ...
of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds.
Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere, where it acts as a greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide and methane. Use of water vapor, as steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
, has been important for cooking, and as a major component in energy production and transport systems since the industrial revolution.
Water vapor is a relatively common atmospheric constituent, present even in the solar atmosphere as well as every planet in the Solar System and many astronomical object
An astronomical object, celestial object, stellar object or heavenly body is a naturally occurring physical entity, association, or structure that exists in the observable universe. In astronomy, the terms ''object'' and ''body'' are often us ...
s including natural satellites, comets and even large asteroid
An asteroid is a minor planet of the inner Solar System. Sizes and shapes of asteroids vary significantly, ranging from 1-meter rocks to a dwarf planet almost 1000 km in diameter; they are rocky, metallic or icy bodies with no atmosphere.
...
s. Likewise the detection of extrasolar water vapor would indicate a similar distribution in other planetary systems. Water vapor can also be indirect evidence supporting the presence of extraterrestrial liquid water in the case of some planetary mass objects.
Properties
Evaporation
Whenever a water molecule leaves a surface and diffuses into a surrounding gas, it is said to have evaporated. Each individual water molecule which transitions between a more associated (liquid) and a less associated (vapor/gas) state does so through the absorption or release of kinetic energy. The aggregate measurement of this kinetic energy transfer is defined as thermal energy and occurs only when there is differential in the temperature of the water molecules. Liquid water that becomes water vapor takes a parcel of heat with it, in a process called evaporative cooling. The amount of water vapor in the air determines how frequently molecules will return to the surface. When a net evaporation occurs, the body of water will undergo a net cooling directly related to the loss of water. In the US, the National Weather Service measures the actual rate of evaporation from a standardized "pan" open water surface outdoors, at various locations nationwide. Others do likewise around the world. The US data is collected and compiled into an annual evaporation map. The measurements range from under 30 to over 120 inches per year. Formulas can be used for calculating the rate of evaporation from a water surface such as a swimming pool. In some countries, the evaporation rate far exceeds the precipitation rate. Evaporative cooling is restricted by atmospheric conditions. Humidity is the amount of water vapor in the air. The vapor content of air is measured with devices known as hygrometers. The measurements are usually expressed as specific humidity or percent relative humidity. The temperatures of the atmosphere and the water surface determine the equilibrium vapor pressure; 100% relative humidity occurs when the partial pressure of water vapor is equal to the equilibrium vapor pressure. This condition is often referred to as complete saturation. Humidity ranges from 0 grams per cubic metre in dry air to 30 grams per cubic metre (0.03 ounce per cubic foot) when the vapor is saturated at 30 °C.Sublimation
Sublimation
Sublimation or sublimate may refer to:
* ''Sublimation'' (album), by Canvas Solaris, 2004
* Sublimation (phase transition), directly from the solid to the gas phase
* Sublimation (psychology), a mature type of defense mechanism
* Sublimate of mer ...
is the process by which water molecules directly leave the surface of ice without first becoming liquid water. Sublimation accounts for the slow mid-winter disappearance of ice and snow at temperatures too low to cause melting. Antarctica shows this effect to a unique degree because it is by far the continent with the lowest rate of precipitation on Earth. As a result, there are large areas where millennial layers of snow have sublimed, leaving behind whatever non-volatile materials they had contained. This is extremely valuable to certain scientific disciplines, a dramatic example being the collection of meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or Natural satellite, moon. When the ...
s that are left exposed in unparalleled numbers and excellent states of preservation.
Sublimation is important in the preparation of certain classes of biological specimens for scanning electron microscopy. Typically the specimens are prepared by cryofixation and freeze-fracture, after which the broken surface is freeze-etched, being eroded by exposure to vacuum till it shows the required level of detail. This technique can display protein molecules, organelle
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as organs are to the body, hence ''organelle,'' the ...
structures and lipid bilayers with very low degrees of distortion.
Condensation
 Water vapor will only condense onto another surface when that surface is cooler than the dew point temperature, or when the water vapor equilibrium in air has been exceeded. When water vapor condenses onto a surface, a net warming occurs on that surface. The water molecule brings heat energy with it. In turn, the temperature of the atmosphere drops slightly. In the atmosphere, condensation produces clouds, fog and precipitation (usually only when facilitated by
Water vapor will only condense onto another surface when that surface is cooler than the dew point temperature, or when the water vapor equilibrium in air has been exceeded. When water vapor condenses onto a surface, a net warming occurs on that surface. The water molecule brings heat energy with it. In turn, the temperature of the atmosphere drops slightly. In the atmosphere, condensation produces clouds, fog and precipitation (usually only when facilitated by cloud condensation nuclei
Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 µm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This c ...
). The dew point of an air parcel is the temperature to which it must cool before water vapor in the air begins to condense. Condensation in the atmosphere forms cloud droplets.
Also, a net condensation of water vapor occurs on surfaces when the temperature of the surface is at or below the dew point temperature of the atmosphere. Deposition is a phase transition separate from condensation which leads to the direct formation of ice from water vapor. Frost
Frost is a thin layer of ice on a solid surface, which forms from water vapor in an above-freezing atmosphere coming in contact with a solid surface whose temperature is below freezing, and resulting in a phase change from water vapor (a gas) ...
and snow are examples of deposition.
There are several mechanisms of cooling by which condensation occurs:
1) Direct loss of heat by conduction or radiation.
2) Cooling from the drop in air pressure which occurs with uplift of air, also known as adiabatic cooling.
Air can be lifted by mountains, which deflect the air upward, by convection, and by cold and warm fronts.
3) Advective cooling - cooling due to horizontal movement of air.
Importance and Uses
* Provides water for plants and animals: Water vapour gets converted to rain and snow that serve as a natural source of water for plants and animals. * Controls evaporation: Excess water vapor in the air decreases the rate of evaporation. * Determines climatic conditions: Excess water vapor in the air produces rain, fog, snow etc. Hence, it determines climatic conditions.Chemical reactions
A number of chemical reactions have water as a product. If the reactions take place at temperatures higher than the dew point of the surrounding air the water will be formed as vapor and increase the local humidity, if below the dew point local condensation will occur. Typical reactions that result in water formation are the burning of hydrogen or hydrocarbons in air or other oxygen containing gas mixtures, or as a result of reactions with oxidizers. In a similar fashion other chemical or physical reactions can take place in the presence of water vapor resulting in new chemicals forming such as rust on iron or steel, polymerization occurring (certain polyurethane foams andcyanoacrylate
Cyanoacrylates are a family of strong fast-acting adhesives with industrial, medical, and household uses. They are derived from ethyl cyanoacrylate and related esters. The cyanoacrylate group in the monomer rapidly polymerizes in the presence ...
glues cure with exposure to atmospheric humidity) or forms changing such as where anhydrous chemicals may absorb enough vapor to form a crystalline structure or alter an existing one, sometimes resulting in characteristic color changes that can be used for measurement
Measurement is the quantification of attributes of an object or event, which can be used to compare with other objects or events.
In other words, measurement is a process of determining how large or small a physical quantity is as compared ...
.
Measurement
Measuring the quantity of water vapor in a medium can be done directly or remotely with varying degrees of accuracy. Remote methods such electromagnetic absorption are possible from satellites above planetary atmospheres. Direct methods may use electronic transducers, moistened thermometers or hygroscopic materials measuring changes in physical properties or dimensions.Impact on air density
Water vapor is lighter or less dense than dry air. At equivalent temperatures it is buoyant with respect to dry air, whereby the density of dry air atstandard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
(273.15 K, 101.325 kPa) is 1.27 g/L and water vapor at standard temperature has a vapor pressure of 0.6 kPa and the much lower density of 0.0048 g/L.
Calculations
 Water vapor and dry air density calculations at 0 °C:
* The molar mass of water is , as calculated from the sum of the atomic masses of its constituent
Water vapor and dry air density calculations at 0 °C:
* The molar mass of water is , as calculated from the sum of the atomic masses of its constituent atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, an ...
.
* The average molar mass of air (approx. 78% nitrogen, N2; 21% oxygen, O2; 1% other gases) is at standard temperature and pressure ( STP).
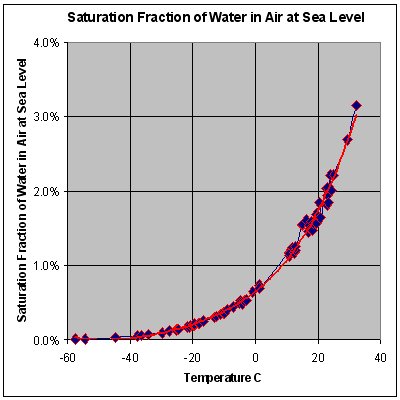
* Obeying Avogadro's Law and the ideal gas law, moist air will have a lower density than dry air. At max. saturation (i. e. rel. humidity = 100% at 0 °C) the density will go down to 28.51 g/mol.
* STP conditions imply a temperature of 0 °C, at which the ability of water to become vapor is very restricted. Its concentration in air is very low at 0 °C. The red line on the chart to the right is the maximum concentration of water vapor expected for a given temperature. The water vapor concentration increases significantly as the temperature rises, approaching 100% (steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
, pure water vapor) at 100 °C. However the difference in densities between air and water vapor would still exist (0.598 vs. 1.27 g/L).
At equal temperatures
At the same temperature, a column of dry air will be denser or heavier than a column of air containing any water vapor, the molar mass of diatomic nitrogen and diatomic oxygen both being greater than the molar mass of water. Thus, any volume of dry air will sink if placed in a larger volume of moist air. Also, a volume of moist air will rise or be buoyant if placed in a larger region of dry air. As the temperature rises the proportion of water vapor in the air increases, and its buoyancy will increase. The increase in buoyancy can have a significant atmospheric impact, giving rise to powerful, moisture rich, upward air currents when the air temperature and sea temperature reaches 25 °C or above. This phenomenon provides a significant driving force for cyclonic and anticyclonic weather systems (typhoons and hurricanes).Respiration and breathing
Water vapor is a by-product of respiration in plants and animals. Its contribution to the pressure, increases as its concentration increases. Itspartial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas ...
contribution to air pressure increases, lowering the partial pressure contribution of the other atmospheric gases (Dalton's Law). The total air pressure must remain constant. The presence of water vapor in the air naturally dilutes or displaces the other air components as its concentration increases.
This can have an effect on respiration. In very warm air (35 °C) the proportion of water vapor is large enough to give rise to the stuffiness that can be experienced in humid jungle conditions or in poorly ventilated buildings.
Lifting gas
Water vapor has lower density than that of air and is therefore buoyant in air but has lower vapor pressure than that of air. When water vapor is used as a lifting gas by athermal airship
A thermal airship is an airship that generates buoyancy by heating air in a large chamber or airship#Envelope, envelope. The lower density of interior hot air compared to cool ambient air causes an upward force on the envelope. This is very simi ...
the water vapor is heated to form steam so that its vapor pressure is greater than the surrounding air pressure in order to maintain the shape of a theoretical "steam balloon", which yields approximately 60% the lift of helium and twice that of hot air.
General discussion
The amount of water vapor in an atmosphere is constrained by the restrictions of partial pressures and temperature. Dew point temperature and relative humidity act as guidelines for the process of water vapor in the water cycle. Energy input, such as sunlight, can trigger more evaporation on an ocean surface or more sublimation on a chunk of ice on top of a mountain. The ''balance'' between condensation and evaporation gives the quantity called vapor partial pressure. The maximum partial pressure (''saturation pressure'') of water vapor in air varies with temperature of the air and water vapor mixture. A variety of empirical formulas exist for this quantity; the most used reference formula is the Goff-Gratch equation for the SVP over liquid water below zero degrees Celsius: : where , temperature of the moist air, is given in units of kelvin, and is given in units of millibars (hectopascal
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined a ...
s).
The formula is valid from about −50 to 102 °C; however there are a very limited number of measurements of the vapor pressure of water over supercooled liquid water. There are a number of other formulae which can be used.
Under certain conditions, such as when the boiling temperature of water is reached, a net evaporation will always occur during standard atmospheric conditions regardless of the percent of relative humidity. This immediate process will dispel massive amounts of water vapor into a cooler atmosphere.
Exhale
Exhalation (or expiration) is the flow of the breath out of an organism. In animals, it is the movement of air from the lungs out of the airways, to the external environment during breathing.
This happens due to elastic properties of the lungs, ...
d air is almost fully at equilibrium with water vapor at the body temperature. In the cold air the exhaled vapor quickly condenses, thus showing up as a fog or mist
Mist is a phenomenon caused by small droplets of water suspended in the cold air, usually by condensation. Physically, it is an example of a dispersion. It is most commonly seen where water vapor in warm, moist air meets sudden cooling, such a ...
of water droplets and as condensation or frost on surfaces. Forcibly condensing these water droplets from exhaled breath is the basis of exhaled breath condensate, an evolving medical diagnostic test.
Controlling water vapor in air is a key concern in the heating, ventilating, and air-conditioning (HVAC) industry. Thermal comfort depends on the moist air conditions. Non-human comfort situations are called refrigeration, and also are affected by water vapor. For example, many food stores, like supermarkets, utilize open chiller cabinets, or ''food cases'', which can significantly lower the water vapor pressure (lowering humidity). This practice delivers several benefits as well as problems.
In Earth's atmosphere
 Gaseous water represents a small but environmentally significant constituent of the
Gaseous water represents a small but environmentally significant constituent of the atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A s ...
. The percentage of water vapor in surface air varies from 0.01% at -42 °C (-44 °F) to 4.24% when the dew point is 30 °C (86 °F). Over 99% of atmospheric water is in the form of vapour, rather than liquid water or ice, and approximately 99.13% of the water vapour is contained in the troposphere. The condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
of water vapor to the liquid or ice phase is responsible for clouds
In meteorology, a cloud is an aerosol consisting of a visible mass of miniature liquid droplets, frozen crystals, or other particles suspended in the atmosphere of a planetary body or similar space. Water or various other chemicals may com ...
, rain, snow, and other precipitation, all of which count among the most significant elements of what we experience as weather. Less obviously, the latent heat of vaporization, which is released to the atmosphere whenever condensation occurs, is one of the most important terms in the atmospheric energy budget on both local and global scales. For example, latent heat release in atmospheric convection is directly responsible for powering destructive storms such as tropical cyclones and severe thunderstorms. Water vapor is an important greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
owing to the presence of the hydroxyl bond which strongly absorbs in the infra-red
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
.
Water vapor is the "working medium" of the atmospheric thermodynamic engine which transforms heat energy from sun irradiation into mechanical energy in the form of winds. Transforming thermal energy into mechanical energy requires an upper and a lower temperature level, as well as a working medium which shuttles forth and back between both. The upper temperature level is given by the soil or water surface of the earth, which absorbs the incoming sun radiation and warms up, evaporating water. The moist and warm air at the ground is lighter than its surroundings and rises up to the upper limit of the troposphere. There the water molecules radiate their thermal energy into outer space, cooling down the surrounding air. The upper atmosphere constitutes the lower temperature level of the atmospheric thermodynamic engine. The water vapor in the now cold air condenses out and falls down to the ground in the form of rain or snow. The now heavier cold and dry air sinks down to ground as well; the atmospheric thermodynamic engine thus establishes a vertical convection, which transports heat from the ground into the upper atmosphere, where the water molecules can radiate it to outer space. Due to the earth's rotation and the resulting Coriolis forces, this vertical atmospheric convection is also converted into a horizontal convection, in the form of cyclones and anticyclones, which transport the water evaporated over the oceans into the interior of the continents, enabling vegetation to grow.
Water in Earth's atmosphere is not merely below its boiling point (100 °C), but at altitude it goes below its freezing point (0 °C), due to water's highly polar attraction. When combined with its quantity, water vapor then has a relevant dew point and frost point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will cond ...
, unlike e. g., carbon dioxide and methane. Water vapor thus has a scale height a fraction of that of the bulk atmosphere, as the water condenses and exits, primarily in the troposphere, the lowest layer of the atmosphere. Carbon dioxide () and methane, being well-mixed in the atmosphere, tend to rise above water vapour. The absorption and emission of both compounds contribute to Earth's emission to space, and thus the planetary greenhouse effect. This greenhouse forcing is directly observable, via distinct spectral features versus water vapor, and observed to be rising with rising levels. Conversely, adding water vapor at high altitudes has a disproportionate impact, which is why jet traffic has a disproportionately high warming effect. Oxidation of methane is also a major source of water vapour in the stratosphere, and adds about 15% to methane's global warming effect.
In the absence of other greenhouse gases, Earth's water vapor would condense to the surface;What is the maximum and minimum distance for the Earth that is compatible with life?/ref>
/ref> this has likely happened, possibly more than once. Scientists thus distinguish between non-condensable (driving) and condensable (driven) greenhouse gases, i.e., the above water vapor feedback.Lacis, A. et al., The role of long-lived greenhouse gases as principal LW control knob that governs the global surface temperature for past and future climate change, Tellus B, vol. 65 p. 19734, 2013
Fog
Fog is a visible aerosol consisting of tiny water droplets or ice crystals suspended in the air at or near the Earth's surface. Reprint from Fog can be considered a type of low-lying cloud usually resembling stratus, and is heavily influ ...
and clouds form through condensation around cloud condensation nuclei
Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 µm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This c ...
. In the absence of nuclei, condensation will only occur at much lower temperatures. Under persistent condensation or deposition, cloud droplets or snowflakes form, which precipitate when they reach a critical mass.
Atmospheric concentration of water vapour is highly variable between locations and times, from 10 ppmv in the coldest air to 5% (50 000 ppmv) in humid tropical air,Wallace, John M. and Peter V. Hobbs''Atmospheric Science: An Introductory Survey''
. Elsevier. Second Edition, 2006. . Page 8. and can be measured with a combination of land observations, weather balloons and satellites. The water content of the atmosphere as a whole is constantly depleted by precipitation. At the same time it is constantly replenished by evaporation, most prominently from oceans, lakes, rivers, and moist earth. Other sources of atmospheric water include combustion, respiration, volcanic eruptions, the transpiration of plants, and various other biological and geological processes. At any given time there is about 1.29 x 1016 litres (3.4 x 1015 gal.) of water in the atmosphere. The atmosphere holds 1 part in 2500 of the fresh water, and 1 part in 100,000 of the total water on Earth. The mean global content of water vapor in the atmosphere is roughly sufficient to cover the surface of the planet with a layer of liquid water about 25 mm deep. The mean annual precipitation for the planet is about 1 metre, a comparison which implies a rapid turnover of water in the air – on average, the residence time of a water molecule in the troposphere is about 9 to 10 days. Global mean water vapour is about 0.25% of the atmosphere by mass and also varies seasonally, in terms of contribution to atmospheric pressure between 2.62 hPa in July and 2.33 hPa in December. IPCC AR6 expresses medium confidence in increase of total water vapour at about 1-2% per decade; it is expected to increase by around 7% per °C of warming. Episodes of surface geothermal activity, such as volcanic eruptions and geysers, release variable amounts of water vapor into the atmosphere. Such eruptions may be large in human terms, and major explosive eruptions may inject exceptionally large masses of water exceptionally high into the atmosphere, but as a percentage of total atmospheric water, the role of such processes is trivial. The relative concentrations of the various gases emitted by volcanoes varies considerably according to the site and according to the particular event at any one site. However, water vapor is consistently the commonest volcanic gas; as a rule, it comprises more than 60% of total emissions during a subaerial eruption. Atmospheric water vapor content is expressed using various measures. These include vapor pressure, specific humidity, mixing ratio, dew point temperature, and relative humidity.
Radar and satellite imaging
Because water molecules absorb microwaves and otherradio wave
Radio waves are a type of electromagnetic radiation with the longest wavelengths in the electromagnetic spectrum, typically with frequencies of 300 gigahertz (GHz) and below. At 300 GHz, the corresponding wavelength is 1 mm (short ...
frequencies, water in the atmosphere attenuates radar signals. In addition, atmospheric water will reflect and refract signals to an extent that depends on whether it is vapor, liquid or solid.
Generally, radar signals lose strength progressively the farther they travel through the troposphere. Different frequencies attenuate at different rates, such that some components of air are opaque to some frequencies and transparent to others. Radio waves used for broadcasting and other communication experience the same effect.
Water vapor reflects radar to a lesser extent than do water's other two phases. In the form of drops and ice crystals, water acts as a prism, which it does not do as an individual molecule; however, the existence of water vapor in the atmosphere causes the atmosphere to act as a giant prism.
A comparison of GOES-12 satellite images shows the distribution of atmospheric water vapor relative to the oceans, clouds and continents of the Earth. Vapor surrounds the planet but is unevenly distributed. The image loop on the right shows monthly average of water vapor content with the units are given in centimeters, which is the precipitable water or equivalent amount of water that could be produced if all the water vapor in the column were to condense. The lowest amounts of water vapor (0 centimeters) appear in yellow, and the highest amounts (6 centimeters) appear in dark blue. Areas of missing data appear in shades of gray. The maps are based on data collected by the Moderate Resolution Imaging Spectroradiometer (MODIS) sensor on NASA's Aqua satellite. The most noticeable pattern in the time series is the influence of seasonal temperature changes and incoming sunlight on water vapor. In the tropics, a band of extremely humid air wobbles north and south of the equator as the seasons change. This band of humidity is part of the Intertropical Convergence Zone
The Intertropical Convergence Zone (ITCZ ), known by sailors as the doldrums or the calms because of its monotonous windless weather, is the area where the northeast and the southeast trade winds converge. It encircles Earth near the thermal e ...
, where the easterly trade winds from each hemisphere converge and produce near-daily thunderstorms and clouds. Farther from the equator, water vapor concentrations are high in the hemisphere experiencing summer and low in the one experiencing winter. Another pattern that shows up in the time series is that water vapor amounts over land areas decrease more in winter months than adjacent ocean areas do. This is largely because air temperatures over land drop more in the winter than temperatures over the ocean. Water vapor condenses more rapidly in colder air.
As water vapor absorbs light in the visible spectral range, its absorption can be used in spectroscopic applications (such as DOAS) to determine the amount of water vapor in the atmosphere. This is done operationally, e.g. from the Global Ozone Monitoring Experiment (GOME) spectrometers on ERS
ERS, Ers or ers may refer to:
Arts and entertainment
* Egyptian Ratscrew or Slap, a card game
* Elevator Repair Service, an American theater ensemble
Economics and finance
* ERS10, a share index of the Serbian Banja Luka Stock Exchange
* Eco ...
(GOME) and MetOp (GOME-2). The weaker water vapor absorption lines in the blue spectral range and further into the UV up to its dissociation limit around 243 nm are mostly based on quantum mechanical calculations and are only partly confirmed by experiments.
Lightning generation
Water vapor plays a key role in lightning production in the atmosphere. From cloud physics, usually clouds are the real generators of static charge as found in Earth's atmosphere. The ability of clouds to hold massive amounts of electrical energy is directly related to the amount of water vapor present in the local system. The amount of water vapor directly controls the permittivity of the air. During times of low humidity, static discharge is quick and easy. During times of higher humidity, fewer static discharges occur. Permittivity and capacitance work hand in hand to produce the megawatt outputs of lightning. After a cloud, for instance, has started its way to becoming a lightning generator, atmospheric water vapor acts as a substance (or insulator) that decreases the ability of the cloud todischarge
Discharge may refer to
Expel or let go
* Discharge, the act of firing a gun
* Discharge, or termination of employment, the end of an employee's duration with an employer
* Military discharge, the release of a member of the armed forces from serv ...
its electrical energy. Over a certain amount of time, if the cloud continues to generate and store more static electricity
Static electricity is an imbalance of electric charges within or on the surface of a material or between materials. The charge remains until it is able to move away by means of an electric current or electrical discharge. Static electricity is na ...
, the barrier that was created by the atmospheric water vapor will ultimately break down from the stored electrical potential energy. This energy will be released to a local oppositely charged region, in the form of lightning. The strength of each discharge is directly related to the atmospheric permittivity, capacitance, and the source's charge generating ability.
Extraterrestrial
Water vapor is common in the Solar System and by extension, otherplanetary system
A planetary system is a set of gravitationally
In physics, gravity () is a fundamental interaction which causes mutual attraction between all things with mass or energy. Gravity is, by far, the weakest of the four fundamental interacti ...
s. Its signature has been detected in the atmospheres of the Sun, occurring in sunspots
Sunspots are phenomena on the Sun's photosphere that appear as temporary spots that are darker than the surrounding areas. They are regions of reduced surface temperature caused by concentrations of magnetic flux that inhibit convection. ...
. The presence of water vapor has been detected in the atmospheres of all seven extraterrestrial planets in the solar system, the Earth's Moon, and the moons of other planets, although typically in only trace amounts.

 Geological formations such as
Geological formations such as cryogeyser
A geyser (, ) is a spring characterized by an intermittent discharge of water ejected turbulently and accompanied by steam. As a fairly rare phenomenon, the formation of geysers is due to particular hydrogeological conditions that exist only in ...
s are thought to exist on the surface of several icy moons ejecting water vapor due to tidal heating and may indicate the presence of substantial quantities of subsurface water. Plumes of water vapor have been detected on Jupiter's moon Europa and are similar to plumes of water vapor detected on Saturn's moon Enceladus. Traces of water vapor have also been detected in the stratosphere of Titan
Titan most often refers to:
* Titan (moon), the largest moon of Saturn
* Titans, a race of deities in Greek mythology
Titan or Titans may also refer to:
Arts and entertainment
Fictional entities
Fictional locations
* Titan in fiction, fictiona ...
. Water vapor has been found to be a major constituent of the atmosphere of dwarf planet, Ceres, largest object in the asteroid belt The detection was made by using the far-infrared abilities of the Herschel Space Observatory
The Herschel Space Observatory was a space observatory built and operated by the European Space Agency (ESA). It was active from 2009 to 2013, and was the largest infrared telescope ever launched until the launch of the James Webb Space Telesc ...
. The finding is unexpected because comets, not asteroids, are typically considered to "sprout jets and plumes." According to one of the scientists, "The lines are becoming more and more blurred between comets and asteroids." Scientists studying Mars hypothesize that if water moves about the planet, it does so as vapor.
The brilliance of comet tails comes largely from water vapor. On approach to the Sun, the ice many comets carry sublimes
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point i ...
to vapor. Knowing a comet's distance from the sun, astronomers may deduce the comet's water content from its brilliance.
Water vapor has also been confirmed outside the Solar System. Spectroscopic analysis of HD 209458 b, an extrasolar planet in the constellation Pegasus, provides the first evidence of atmospheric water vapor beyond the Solar System. A star called CW Leonis was found to have a ring of vast quantities of water vapor circling the aging, massive star
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked ...
. A NASA satellite designed to study chemicals in interstellar gas clouds, made the discovery with an onboard spectrometer. Most likely, "the water vapor was vaporized from the surfaces of orbiting comets." Other exoplanets with evidence of water vapor include HAT-P-11b and K2-18b.
See also
*Air density
The density of air or atmospheric density, denoted '' ρ'', is the mass per unit volume of Earth's atmosphere. Air density, like air pressure, decreases with increasing altitude. It also changes with variation in atmospheric pressure, temperature a ...
* Atmospheric river
* Boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
* Condensation in aerosol dynamics
* Deposition
* Earth's atmosphere
* Eddy covariance
* Equation of state
* Evaporative cooler
* Fog
Fog is a visible aerosol consisting of tiny water droplets or ice crystals suspended in the air at or near the Earth's surface. Reprint from Fog can be considered a type of low-lying cloud usually resembling stratus, and is heavily influ ...
* Frost
Frost is a thin layer of ice on a solid surface, which forms from water vapor in an above-freezing atmosphere coming in contact with a solid surface whose temperature is below freezing, and resulting in a phase change from water vapor (a gas) ...
* Gas laws
* Gibbs free energy
* Gibbs phase rule
* Greenhouse gas
A greenhouse gas (GHG or GhG) is a gas that Absorption (electromagnetic radiation), absorbs and Emission (electromagnetic radiation), emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse ...
* Heat capacity
* Heat of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
* Humidity
* Hygrometer
* Ideal gas
* Kinetic theory of gases
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and enter ...
* Latent heat
* Latent heat flux
* Microwave radiometer
* Phase of matter
* Saturation vapor density
The saturation vapor density (SVD) is the maximum density of water vapor in air at a given temperature. The concept is related to saturation vapor pressure (SVP). It can be used to calculate exact quantity of water vapor in the air from a relative ...
* Steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
* Sublimation
Sublimation or sublimate may refer to:
* ''Sublimation'' (album), by Canvas Solaris, 2004
* Sublimation (phase transition), directly from the solid to the gas phase
* Sublimation (psychology), a mature type of defense mechanism
* Sublimate of mer ...
* Superheating
In thermodynamics, superheating (sometimes referred to as boiling retardation, or boiling delay) is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling. This is a so-called ''metastable state ...
* Supersaturation
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a liqu ...
* Thermodynamics
* Troposphere
* Vapor pressure
References
Bibliography
* * * * * * * * * * *External links
National Science Digital Library – Water Vapor
Calculate the condensation of your exhaled breath
Water Vapor Myths: A Brief Tutorial
– PhyMetrix {{Authority control Greenhouse gases Atmospheric thermodynamics Forms of water Water in gas Psychrometrics Articles containing video clips