Vinylcyclopropane-cyclopentene Rearrangement on:
[Wikipedia]
[Google]
[Amazon]
The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a  Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.
Due to its ability to form
Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.
Due to its ability to form
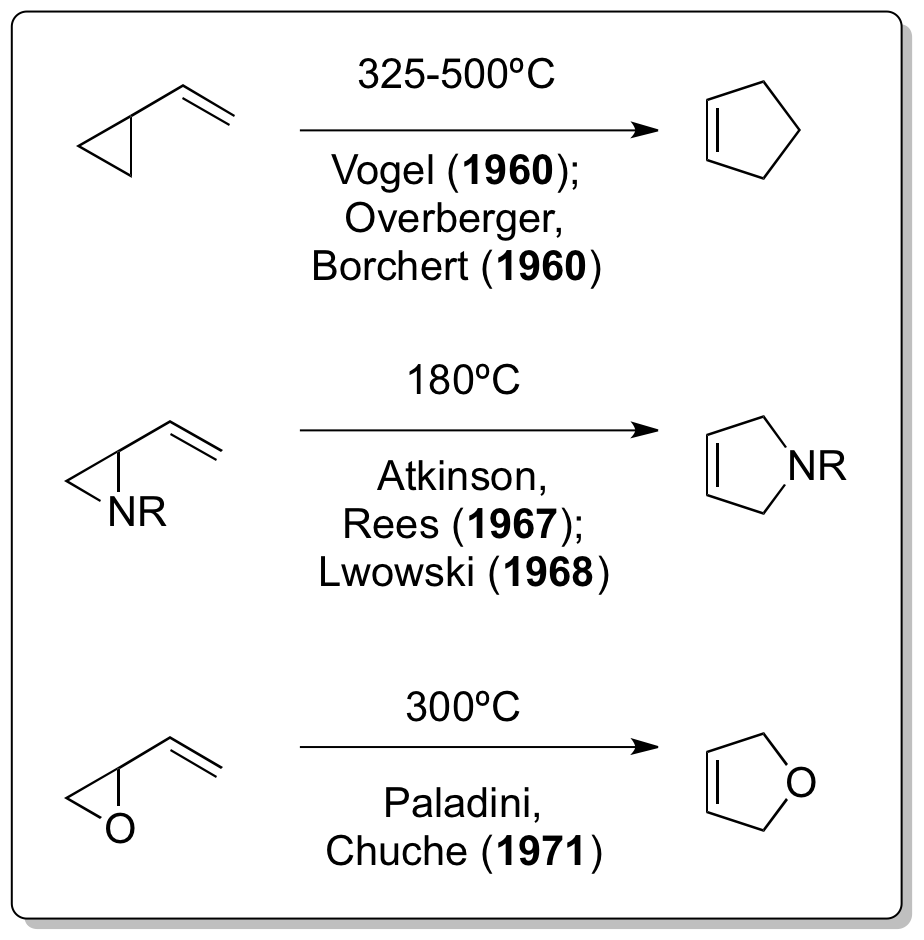
 The corresponding all-carbon version of the reaction was independently reported by Emanuel Vogel and Overberger & Borchert just one year after the Neureiter publication appeared.
Doering, although interacting with Humble Oil and Refining - and therefore also with Neureiter - as a consultant, in a 1963 publication stated the following : ''"Credit for discovery that vinylcyclopropane rearranges to cyclopentene is due to Overberger and Borchert, and Vogel et al., who appear to have developed several examples of the rearrangement independently."''
The development of further vinylcyclopropane rearrangement variants didn't take long as demonstrated by Atkinson & Rees in 1967, Lwowski in 1968. and Paladini & Chuche in 1971.
The corresponding all-carbon version of the reaction was independently reported by Emanuel Vogel and Overberger & Borchert just one year after the Neureiter publication appeared.
Doering, although interacting with Humble Oil and Refining - and therefore also with Neureiter - as a consultant, in a 1963 publication stated the following : ''"Credit for discovery that vinylcyclopropane rearranges to cyclopentene is due to Overberger and Borchert, and Vogel et al., who appear to have developed several examples of the rearrangement independently."''
The development of further vinylcyclopropane rearrangement variants didn't take long as demonstrated by Atkinson & Rees in 1967, Lwowski in 1968. and Paladini & Chuche in 1971.
 It is remarkable that the classical vinylcyclopropane rearrangement was discovered after two of its heteroatom variants had already been reported for almost 30 years and 12 years, respectively. Although it is believed that the vinylcyclopropane rearrangement must have occurred during Nikolay Demyanov's preparation of vinylcyclopropane by
It is remarkable that the classical vinylcyclopropane rearrangement was discovered after two of its heteroatom variants had already been reported for almost 30 years and 12 years, respectively. Although it is believed that the vinylcyclopropane rearrangement must have occurred during Nikolay Demyanov's preparation of vinylcyclopropane by  This last reaction type is also known as the Cloke–Wilson Rearrangement
This last reaction type is also known as the Cloke–Wilson Rearrangement
 The discussion on whether the vinylcyclopropane rearrangement proceeds via a fully concerted or a two-step, non-concerted mechanism was given further careful consideration when Woodward and Hoffmann used the vinylcyclopropane rearrangement to exemplify ,3sigmatropic concerted alkyl shifts in 1969. They hypothesized that if a concerted mechanism was operative the consequences of orbital-symmetry controlled factors would only allow the formation of certain products. According to their analysis of a vinylcyclopropane substituted with three R groups the
antarafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to the ''ar'' cyclopentene and the suprafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to cyclopentene ''si'' are symmetry allowed whereas the suprafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to cyclopentene ''sr'' and the antarafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to the ''ai'' cyclopentene are symmetry-forbidden. It is important to note that Woodward and Hoffmann based their analysis solely on the principles of the conservation of orbital symmetry theory without however making any mechanistic or stereochemical prediction.
The discussion on whether the vinylcyclopropane rearrangement proceeds via a fully concerted or a two-step, non-concerted mechanism was given further careful consideration when Woodward and Hoffmann used the vinylcyclopropane rearrangement to exemplify ,3sigmatropic concerted alkyl shifts in 1969. They hypothesized that if a concerted mechanism was operative the consequences of orbital-symmetry controlled factors would only allow the formation of certain products. According to their analysis of a vinylcyclopropane substituted with three R groups the
antarafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to the ''ar'' cyclopentene and the suprafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to cyclopentene ''si'' are symmetry allowed whereas the suprafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to cyclopentene ''sr'' and the antarafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to the ''ai'' cyclopentene are symmetry-forbidden. It is important to note that Woodward and Hoffmann based their analysis solely on the principles of the conservation of orbital symmetry theory without however making any mechanistic or stereochemical prediction.
 The attention directed towards the vinylcyclopropane rearrangement by Woodward and Hoffmann as a representative example for ,3carbon shifts clearly enhanced the interest in this reaction. Furthermore, their analysis revealed potential experiments that would allow to distinguish between a concerted or stepwise mechanism. The stereochemical consequences of a concerted reaction pathway on the reaction outcome suggested an experiment where one would correlate the obtained reaction stereochemistry with the predicted reaction stereochemistry for a model substrate. Observing the formation of ''ai''- and ''sr''-cyclopentene products would support the notion that a stepwise, non-concerted mechanism is operative whereas their absence would point towards a fully concerted mechanism. As it turned out finding an appropriate substituted model substrate to study the stereochemical outcome of the vinylcyclopropane rearrangement was much more challenging than initially thought since side reaction such as the homodienyl ,5hydrogen shifts and more so thermal stereomutations tend to scramble stereochemical distinctions much faster than rearrangements lead to the cyclopentene products.
The attention directed towards the vinylcyclopropane rearrangement by Woodward and Hoffmann as a representative example for ,3carbon shifts clearly enhanced the interest in this reaction. Furthermore, their analysis revealed potential experiments that would allow to distinguish between a concerted or stepwise mechanism. The stereochemical consequences of a concerted reaction pathway on the reaction outcome suggested an experiment where one would correlate the obtained reaction stereochemistry with the predicted reaction stereochemistry for a model substrate. Observing the formation of ''ai''- and ''sr''-cyclopentene products would support the notion that a stepwise, non-concerted mechanism is operative whereas their absence would point towards a fully concerted mechanism. As it turned out finding an appropriate substituted model substrate to study the stereochemical outcome of the vinylcyclopropane rearrangement was much more challenging than initially thought since side reaction such as the homodienyl ,5hydrogen shifts and more so thermal stereomutations tend to scramble stereochemical distinctions much faster than rearrangements lead to the cyclopentene products.
 Even though deconvolution of the complex kinetic scenarios underlying these rearrangements was difficult there have been several studies reported where exact and explicit deconvolutions of kinetic and stereochemical raw data to account for the stereochemical contributions arising from competitive stereomutations was possible.
Thereby rate constants for all four stereochemically distinct pathways of the vinylcyclopropane rearrangement could be determined.
Even though deconvolution of the complex kinetic scenarios underlying these rearrangements was difficult there have been several studies reported where exact and explicit deconvolutions of kinetic and stereochemical raw data to account for the stereochemical contributions arising from competitive stereomutations was possible.
Thereby rate constants for all four stereochemically distinct pathways of the vinylcyclopropane rearrangement could be determined.
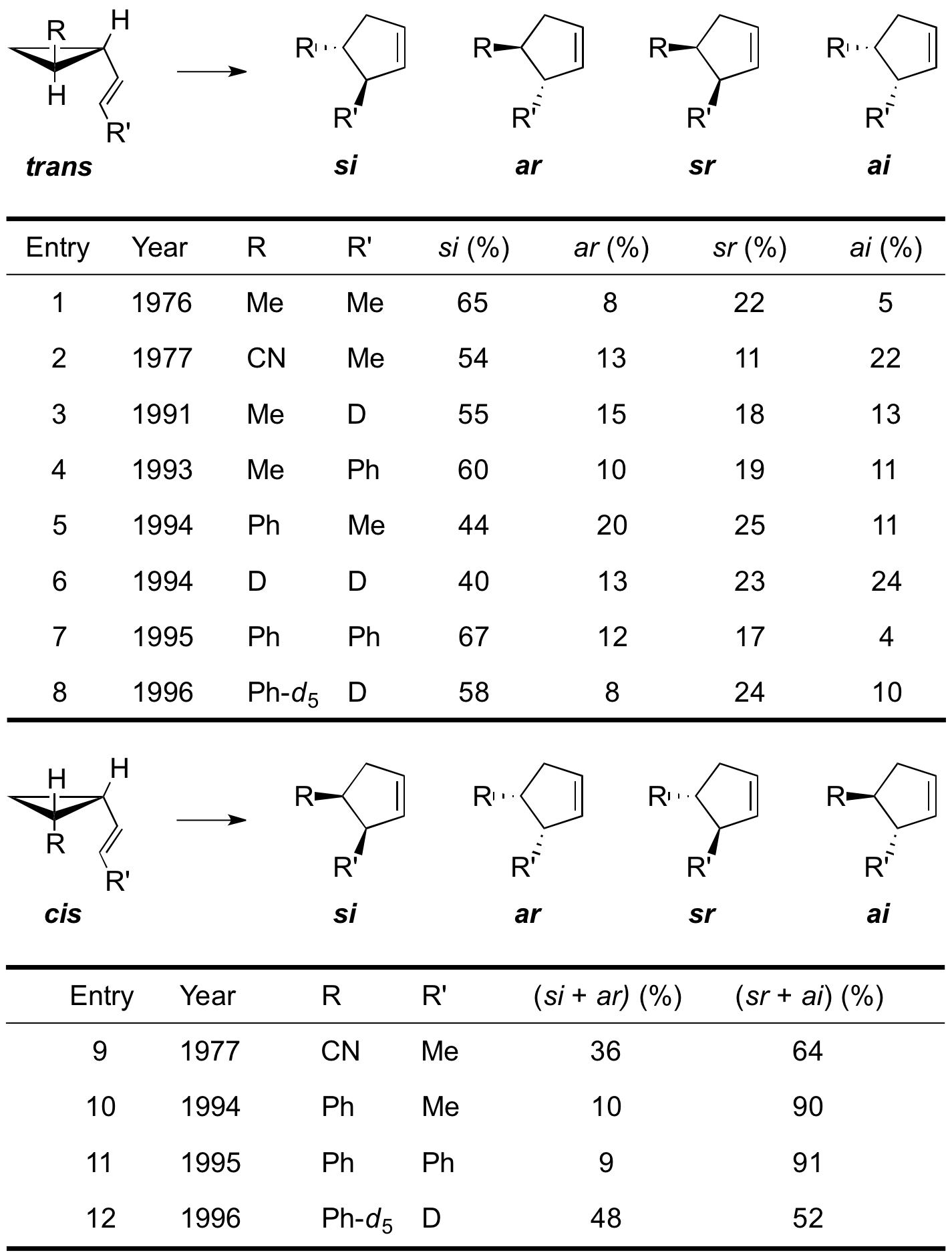 The data clearly indicated that the mechanistic preferences of the rearrangements are system dependent. Whereas ''trans''-vinylcyclopropanes tend to form more of the symmetry-allowed ''ar''- and ''si''-cyclopentenes supportive of a concerted mechanism, the ''cis''-vinylcyclopropanes preferentially yield the symmetry-forbidden ''ai''- and ''sr''- products suggesting a more stepwise, diradical mechanism. The influence of substituent effects on the reaction stereochemistry also becomes apparent from the data. Substituents with increased radical stabilizing ability not only lower the rearrangements activation energy but also reclosure of the initially formed diradical species becomes slower relative to the rate of cyclopentene formation resulting in an overall more concerted mechanism with less stereomutation (e.g. entry 6 & 7). In all cases though all the four products were formed indicating that both orbital-symmetry controlled pericyclic, as well as diradical-mediated two-step mechanisms are operative either way. The data is consistent with the formation of biradical species on a relatively flat potential energy surface allowing for restricted conformational flexibility before the products are formed. The amount of conformational flexibility and therefore conformational evolution accessible to the diradical species before forming product depends on the constitution of the potential energy surface.
This notion is also supported by computational work. One transition state with a high diradicaloid character was found. Following the potential energy surface of the lowest energy path of the reaction it was found that a very shallow regime allows the diradical species to undergo conformational changes and stereoisomerization reactions with minor energetic consequences. Furthermore, it was shown that substituents can favor stereoselective pathways by destabilizing species that allow stereochemical scrambling.
The data clearly indicated that the mechanistic preferences of the rearrangements are system dependent. Whereas ''trans''-vinylcyclopropanes tend to form more of the symmetry-allowed ''ar''- and ''si''-cyclopentenes supportive of a concerted mechanism, the ''cis''-vinylcyclopropanes preferentially yield the symmetry-forbidden ''ai''- and ''sr''- products suggesting a more stepwise, diradical mechanism. The influence of substituent effects on the reaction stereochemistry also becomes apparent from the data. Substituents with increased radical stabilizing ability not only lower the rearrangements activation energy but also reclosure of the initially formed diradical species becomes slower relative to the rate of cyclopentene formation resulting in an overall more concerted mechanism with less stereomutation (e.g. entry 6 & 7). In all cases though all the four products were formed indicating that both orbital-symmetry controlled pericyclic, as well as diradical-mediated two-step mechanisms are operative either way. The data is consistent with the formation of biradical species on a relatively flat potential energy surface allowing for restricted conformational flexibility before the products are formed. The amount of conformational flexibility and therefore conformational evolution accessible to the diradical species before forming product depends on the constitution of the potential energy surface.
This notion is also supported by computational work. One transition state with a high diradicaloid character was found. Following the potential energy surface of the lowest energy path of the reaction it was found that a very shallow regime allows the diradical species to undergo conformational changes and stereoisomerization reactions with minor energetic consequences. Furthermore, it was shown that substituents can favor stereoselective pathways by destabilizing species that allow stereochemical scrambling.
 Only a year later Simpson and co-workers demonstrated that also simple methoxy-substituted vinylcyclopropanes show significantly faster reaction rates allowing the rearrangement to take place at 220 °C.
Only a year later Simpson and co-workers demonstrated that also simple methoxy-substituted vinylcyclopropanes show significantly faster reaction rates allowing the rearrangement to take place at 220 °C.
 A big improvement came in the mid-1970s from Barry M. Trost's group. It was found that siloxyvinylcyclopropanes as well as the analogous sulfinylvinylcyclopropanes could be used as substrates to build annulated
A big improvement came in the mid-1970s from Barry M. Trost's group. It was found that siloxyvinylcyclopropanes as well as the analogous sulfinylvinylcyclopropanes could be used as substrates to build annulated  Paquette demonstrated that vinylcyclopropane rearrangements can also be mediated photochemically. In a particularly intriguing example he was able to show that vinylcyclopropanes embedded within a
Paquette demonstrated that vinylcyclopropane rearrangements can also be mediated photochemically. In a particularly intriguing example he was able to show that vinylcyclopropanes embedded within a  Further reaction improvement came when Hudlicky and Brown proved that vinylcyclopropane rearrangements are amenable to
Further reaction improvement came when Hudlicky and Brown proved that vinylcyclopropane rearrangements are amenable to 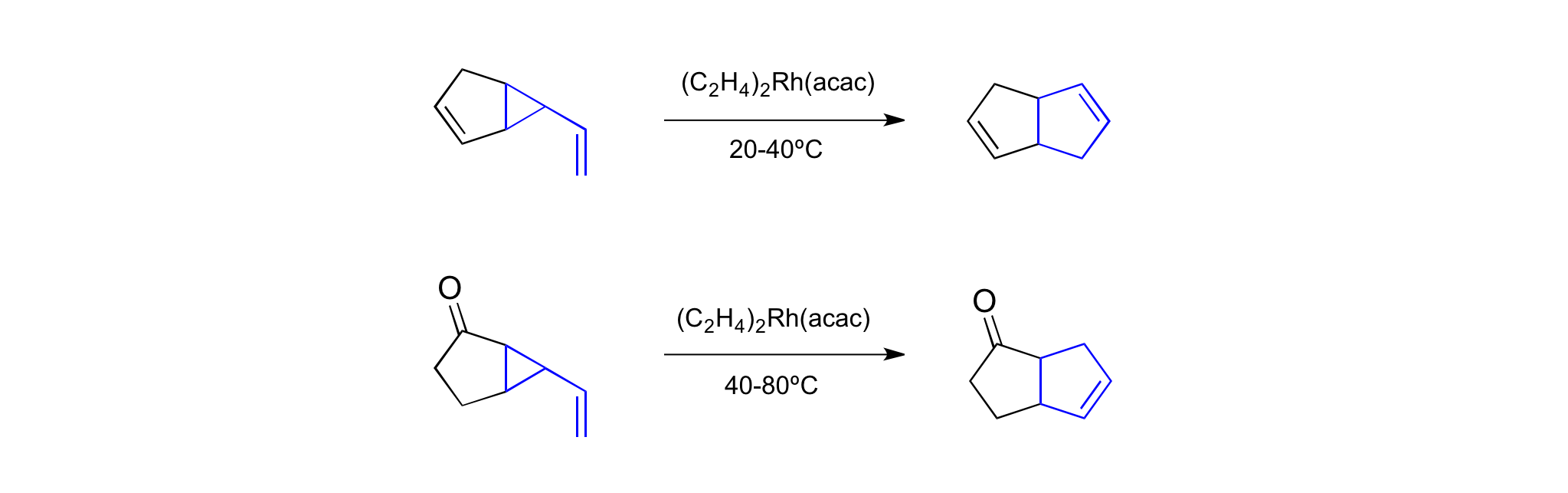 Analogous to the rate acceleration observed in the anionic-oxy-
Analogous to the rate acceleration observed in the anionic-oxy- Another intriguing result was reported by Larsen in 1988. He was able to promote vinylcyclopropane rearrangements with substrates such as the one shown in the reaction below at temperatures as low as -78 °C. The substrates were generated ''in situ'' upon ringcontracting thiocarbonyl Diels-Alder adducts under basic conditions. This methodology allowed the formation of numerous highly functionalized cyclopentenes in a
Another intriguing result was reported by Larsen in 1988. He was able to promote vinylcyclopropane rearrangements with substrates such as the one shown in the reaction below at temperatures as low as -78 °C. The substrates were generated ''in situ'' upon ringcontracting thiocarbonyl Diels-Alder adducts under basic conditions. This methodology allowed the formation of numerous highly functionalized cyclopentenes in a  Another low temperature vinylcyclopropane rearrangement was obtained by the Hudlicky group. The scope of this particular methodology is impressively broad and allows the formation of various -5 as well as -6carbon scaffolds.
Another low temperature vinylcyclopropane rearrangement was obtained by the Hudlicky group. The scope of this particular methodology is impressively broad and allows the formation of various -5 as well as -6carbon scaffolds.





ring expansion reaction
Ring expansion and ring contraction reactions in the course of organic synthesis refer to a set of reactions which can lead to the expansion or contraction of an existing Ring (chemistry), ring. This often makes it possible to access structures ...
, converting a vinyl-substituted cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself ...
ring into a cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of t ...
ring.
 Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.
Due to its ability to form
Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.
Due to its ability to form cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of t ...
rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.
Origins and history
In 1959, a young research chemist with Humble Oil and Refining (Esso
Esso () is a trading name for ExxonMobil. Originally, the name was primarily used by its predecessor Standard Oil of New Jersey after the breakup of the original Standard Oil company in 1911. The company adopted the name "Esso" (the phonetic p ...
, now Exxon
ExxonMobil Corporation (commonly shortened to Exxon) is an American multinational oil and gas corporation headquartered in Irving, Texas. It is the largest direct descendant of John D. Rockefeller's Standard Oil, and was formed on November 30, ...
) named Norman P. Neureiter was instructed to find new uses for the excess butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
produced from one of the refinery processes. Discussions about carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
chemistry with one of the company's most respectable consultants at the time, William von Eggers Doering
William von Eggers Doering (June 22, 1917 – January 3, 2011) was the Mallinckrodt Professor of Chemistry at Harvard University. Before Harvard, he taught at Columbia University, Columbia (1942–1952) and Yale (1952–1968).
Doering was born ...
, then a professor at Yale
Yale University is a private research university in New Haven, Connecticut. Established in 1701 as the Collegiate School, it is the third-oldest institution of higher education in the United States and among the most prestigious in the wor ...
, led the young Ph.D. graduate from Northwestern University
Northwestern University is a private research university in Evanston, Illinois. Founded in 1851, Northwestern is the oldest chartered university in Illinois and is ranked among the most prestigious academic institutions in the world.
Charte ...
to follow a recent procedure combining both, carbenes and butadiene.
In particular the procedure described the reaction of 1,3-butadiene with carbenes generated from the action of base on chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to ...
or bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, ...
, which had been studied previously by Doering. Neureiter then took the resulting 1,1-dichloro-2,2-dimethylcyclopropane and under pyrolysis conditions (above 400 °C) discovered a rearrangement to 4,4-dichlorocyclopentene which today is considered to be the first thermal vinylcyclopropane-cyclopentene rearrangement in history.
 The corresponding all-carbon version of the reaction was independently reported by Emanuel Vogel and Overberger & Borchert just one year after the Neureiter publication appeared.
Doering, although interacting with Humble Oil and Refining - and therefore also with Neureiter - as a consultant, in a 1963 publication stated the following : ''"Credit for discovery that vinylcyclopropane rearranges to cyclopentene is due to Overberger and Borchert, and Vogel et al., who appear to have developed several examples of the rearrangement independently."''
The development of further vinylcyclopropane rearrangement variants didn't take long as demonstrated by Atkinson & Rees in 1967, Lwowski in 1968. and Paladini & Chuche in 1971.
The corresponding all-carbon version of the reaction was independently reported by Emanuel Vogel and Overberger & Borchert just one year after the Neureiter publication appeared.
Doering, although interacting with Humble Oil and Refining - and therefore also with Neureiter - as a consultant, in a 1963 publication stated the following : ''"Credit for discovery that vinylcyclopropane rearranges to cyclopentene is due to Overberger and Borchert, and Vogel et al., who appear to have developed several examples of the rearrangement independently."''
The development of further vinylcyclopropane rearrangement variants didn't take long as demonstrated by Atkinson & Rees in 1967, Lwowski in 1968. and Paladini & Chuche in 1971.
 It is remarkable that the classical vinylcyclopropane rearrangement was discovered after two of its heteroatom variants had already been reported for almost 30 years and 12 years, respectively. Although it is believed that the vinylcyclopropane rearrangement must have occurred during Nikolay Demyanov's preparation of vinylcyclopropane by
It is remarkable that the classical vinylcyclopropane rearrangement was discovered after two of its heteroatom variants had already been reported for almost 30 years and 12 years, respectively. Although it is believed that the vinylcyclopropane rearrangement must have occurred during Nikolay Demyanov's preparation of vinylcyclopropane by Hofmann elimination
Hofmann elimination is an elimination reaction of an amine to form alkenes. The least stable alkene (the one with the least number of substituents on the carbons of the double bond), called the Hofmann product, is formed. This tendency, known as ...
at elevated temperatures in 1922, the cyclopropylimine-pyrroline rearrangement by Cloke in 1929 and Wilson's cyclopropylcarbaldehyde-2,3-dihydrofuran rearrangement in 1947 are really the only examples of vinylcyclopropane-like rearrangements.
 This last reaction type is also known as the Cloke–Wilson Rearrangement
This last reaction type is also known as the Cloke–Wilson Rearrangement
Mechanism
The mechanistic discussion on whether the vinylcyclopropane rearrangement proceeds through a diradical-mediated two-step or a fully concerted orbital-symmetry-controlled mechanism has been going on for more than half a century. Kinetic data together with the secondary kinetic isotope effects observed at the vinyl terminus of the vinylcyclopropane suggest a concerted mechanism whereas product distribution indicates a stepwise-diradical mechanism. In the 1960s, shortly after the rearrangement was discovered, it was established that theactivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
for the vinylcyclopropane rearrangement is around 50 kcal/mol. The kinetic data obtained for this rearrangement were consistent with a concerted mechanism where cleavage of the cyclopropyl carbon-carbon bond was rate-limiting. Albeit a concerted mechanism seemed likely it was shortly recognized that the activation energy to break the carbon-carbon bond in unsubstituted cyclopropane was with 63 kcal/mol exactly 13 kcal/mol higher in energy than the parent activation energy, a difference remarkably similar to the resonance energy of the allyl radical. Immediately people started to appreciate the possibility for a diradical intermediate arising from homolytic cleavage of the weak C1-C2-cyclopropane bond under thermal conditions.
 The discussion on whether the vinylcyclopropane rearrangement proceeds via a fully concerted or a two-step, non-concerted mechanism was given further careful consideration when Woodward and Hoffmann used the vinylcyclopropane rearrangement to exemplify ,3sigmatropic concerted alkyl shifts in 1969. They hypothesized that if a concerted mechanism was operative the consequences of orbital-symmetry controlled factors would only allow the formation of certain products. According to their analysis of a vinylcyclopropane substituted with three R groups the
antarafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to the ''ar'' cyclopentene and the suprafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to cyclopentene ''si'' are symmetry allowed whereas the suprafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to cyclopentene ''sr'' and the antarafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to the ''ai'' cyclopentene are symmetry-forbidden. It is important to note that Woodward and Hoffmann based their analysis solely on the principles of the conservation of orbital symmetry theory without however making any mechanistic or stereochemical prediction.
The discussion on whether the vinylcyclopropane rearrangement proceeds via a fully concerted or a two-step, non-concerted mechanism was given further careful consideration when Woodward and Hoffmann used the vinylcyclopropane rearrangement to exemplify ,3sigmatropic concerted alkyl shifts in 1969. They hypothesized that if a concerted mechanism was operative the consequences of orbital-symmetry controlled factors would only allow the formation of certain products. According to their analysis of a vinylcyclopropane substituted with three R groups the
antarafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to the ''ar'' cyclopentene and the suprafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to cyclopentene ''si'' are symmetry allowed whereas the suprafacial ,3shift of bond 1,2 to C-5, with retention at C-2, leading to cyclopentene ''sr'' and the antarafacial ,3shift of bond 1,2 to C-5, with inversion at C-2, leading to the ''ai'' cyclopentene are symmetry-forbidden. It is important to note that Woodward and Hoffmann based their analysis solely on the principles of the conservation of orbital symmetry theory without however making any mechanistic or stereochemical prediction.
 The attention directed towards the vinylcyclopropane rearrangement by Woodward and Hoffmann as a representative example for ,3carbon shifts clearly enhanced the interest in this reaction. Furthermore, their analysis revealed potential experiments that would allow to distinguish between a concerted or stepwise mechanism. The stereochemical consequences of a concerted reaction pathway on the reaction outcome suggested an experiment where one would correlate the obtained reaction stereochemistry with the predicted reaction stereochemistry for a model substrate. Observing the formation of ''ai''- and ''sr''-cyclopentene products would support the notion that a stepwise, non-concerted mechanism is operative whereas their absence would point towards a fully concerted mechanism. As it turned out finding an appropriate substituted model substrate to study the stereochemical outcome of the vinylcyclopropane rearrangement was much more challenging than initially thought since side reaction such as the homodienyl ,5hydrogen shifts and more so thermal stereomutations tend to scramble stereochemical distinctions much faster than rearrangements lead to the cyclopentene products.
The attention directed towards the vinylcyclopropane rearrangement by Woodward and Hoffmann as a representative example for ,3carbon shifts clearly enhanced the interest in this reaction. Furthermore, their analysis revealed potential experiments that would allow to distinguish between a concerted or stepwise mechanism. The stereochemical consequences of a concerted reaction pathway on the reaction outcome suggested an experiment where one would correlate the obtained reaction stereochemistry with the predicted reaction stereochemistry for a model substrate. Observing the formation of ''ai''- and ''sr''-cyclopentene products would support the notion that a stepwise, non-concerted mechanism is operative whereas their absence would point towards a fully concerted mechanism. As it turned out finding an appropriate substituted model substrate to study the stereochemical outcome of the vinylcyclopropane rearrangement was much more challenging than initially thought since side reaction such as the homodienyl ,5hydrogen shifts and more so thermal stereomutations tend to scramble stereochemical distinctions much faster than rearrangements lead to the cyclopentene products.
 Even though deconvolution of the complex kinetic scenarios underlying these rearrangements was difficult there have been several studies reported where exact and explicit deconvolutions of kinetic and stereochemical raw data to account for the stereochemical contributions arising from competitive stereomutations was possible.
Thereby rate constants for all four stereochemically distinct pathways of the vinylcyclopropane rearrangement could be determined.
Even though deconvolution of the complex kinetic scenarios underlying these rearrangements was difficult there have been several studies reported where exact and explicit deconvolutions of kinetic and stereochemical raw data to account for the stereochemical contributions arising from competitive stereomutations was possible.
Thereby rate constants for all four stereochemically distinct pathways of the vinylcyclopropane rearrangement could be determined.
 The data clearly indicated that the mechanistic preferences of the rearrangements are system dependent. Whereas ''trans''-vinylcyclopropanes tend to form more of the symmetry-allowed ''ar''- and ''si''-cyclopentenes supportive of a concerted mechanism, the ''cis''-vinylcyclopropanes preferentially yield the symmetry-forbidden ''ai''- and ''sr''- products suggesting a more stepwise, diradical mechanism. The influence of substituent effects on the reaction stereochemistry also becomes apparent from the data. Substituents with increased radical stabilizing ability not only lower the rearrangements activation energy but also reclosure of the initially formed diradical species becomes slower relative to the rate of cyclopentene formation resulting in an overall more concerted mechanism with less stereomutation (e.g. entry 6 & 7). In all cases though all the four products were formed indicating that both orbital-symmetry controlled pericyclic, as well as diradical-mediated two-step mechanisms are operative either way. The data is consistent with the formation of biradical species on a relatively flat potential energy surface allowing for restricted conformational flexibility before the products are formed. The amount of conformational flexibility and therefore conformational evolution accessible to the diradical species before forming product depends on the constitution of the potential energy surface.
This notion is also supported by computational work. One transition state with a high diradicaloid character was found. Following the potential energy surface of the lowest energy path of the reaction it was found that a very shallow regime allows the diradical species to undergo conformational changes and stereoisomerization reactions with minor energetic consequences. Furthermore, it was shown that substituents can favor stereoselective pathways by destabilizing species that allow stereochemical scrambling.
The data clearly indicated that the mechanistic preferences of the rearrangements are system dependent. Whereas ''trans''-vinylcyclopropanes tend to form more of the symmetry-allowed ''ar''- and ''si''-cyclopentenes supportive of a concerted mechanism, the ''cis''-vinylcyclopropanes preferentially yield the symmetry-forbidden ''ai''- and ''sr''- products suggesting a more stepwise, diradical mechanism. The influence of substituent effects on the reaction stereochemistry also becomes apparent from the data. Substituents with increased radical stabilizing ability not only lower the rearrangements activation energy but also reclosure of the initially formed diradical species becomes slower relative to the rate of cyclopentene formation resulting in an overall more concerted mechanism with less stereomutation (e.g. entry 6 & 7). In all cases though all the four products were formed indicating that both orbital-symmetry controlled pericyclic, as well as diradical-mediated two-step mechanisms are operative either way. The data is consistent with the formation of biradical species on a relatively flat potential energy surface allowing for restricted conformational flexibility before the products are formed. The amount of conformational flexibility and therefore conformational evolution accessible to the diradical species before forming product depends on the constitution of the potential energy surface.
This notion is also supported by computational work. One transition state with a high diradicaloid character was found. Following the potential energy surface of the lowest energy path of the reaction it was found that a very shallow regime allows the diradical species to undergo conformational changes and stereoisomerization reactions with minor energetic consequences. Furthermore, it was shown that substituents can favor stereoselective pathways by destabilizing species that allow stereochemical scrambling.
Methodology development
Arguably the biggest drawback of the vinylcyclopropane rearrangement as a synthetic method is its intrinsically high activation barrier resulting in very high reaction temperatures (500-600 °C). Not only do these high temperatures allow side reactions with similar activation energies, such as homodienyl- ,5hydrogen shifts, to occur but also do they significantly limit the functional groups tolerated in the substrates. It was well recognized by the chemical community that in order for this reaction to become a useful synthetic method, hopefully applicable in complex natural product settings at some point, some reaction development had to be done. Some of the earliest attempts to improve the vinylcyclopropane rearrangement as a synthetic method came from theCorey
Corey is a masculine given name and a surname. It is a masculine version of name Cora, which has Greek origins and is the maiden name of the goddess Persephone. The name also can have origins from the Gaelic word ''coire'', which means "in a caul ...
group in 1972. They found that the reaction temperature could be lowered drastically when the cyclopropane ring contained a dithiane
A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene bridges (-- units) are replaced by sulfur centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane.
1,3- ...
group. Even though the dithiane-substituted vinylcyclopropane substrates required two synthetic steps starting from the corresponding 1,3-dienes the method proved itself successful for the synthesis of a variety of substituted cyclopentenes. The immediate rearrangement products could be easily converted to the corresponding cyclopentenones.
 Only a year later Simpson and co-workers demonstrated that also simple methoxy-substituted vinylcyclopropanes show significantly faster reaction rates allowing the rearrangement to take place at 220 °C.
Only a year later Simpson and co-workers demonstrated that also simple methoxy-substituted vinylcyclopropanes show significantly faster reaction rates allowing the rearrangement to take place at 220 °C.
 A big improvement came in the mid-1970s from Barry M. Trost's group. It was found that siloxyvinylcyclopropanes as well as the analogous sulfinylvinylcyclopropanes could be used as substrates to build annulated
A big improvement came in the mid-1970s from Barry M. Trost's group. It was found that siloxyvinylcyclopropanes as well as the analogous sulfinylvinylcyclopropanes could be used as substrates to build annulated cyclopentene
Cyclopentene is a chemical compound with the formula . It is a colorless liquid with a petrol-like odor. It has few applications, and thus is mainly used as a minor component of gasoline, present in concentrations of less than 1%. It is one of t ...
structures. Albeit these reactions still required reaction temperatures above 300 °C they were able to make useful products arising from the annulation
In organic chemistry annulation (from the Latin ''anellus'' for "little ring"; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule.
:
Examples are the Robinson annulation, Danheiser annulation and cert ...
of cyclopentene to a present ring system.
 Paquette demonstrated that vinylcyclopropane rearrangements can also be mediated photochemically. In a particularly intriguing example he was able to show that vinylcyclopropanes embedded within a
Paquette demonstrated that vinylcyclopropane rearrangements can also be mediated photochemically. In a particularly intriguing example he was able to show that vinylcyclopropanes embedded within a cyclooctane
Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general.
Cyclooctane has a camphoraceous odor.
Conformatio ...
core can be converted to the corresponding -5fused ring systems.
 Further reaction improvement came when Hudlicky and Brown proved that vinylcyclopropane rearrangements are amenable to
Further reaction improvement came when Hudlicky and Brown proved that vinylcyclopropane rearrangements are amenable to transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
catalysts. Using a Rh(I) acetate catalyst they were able to promote rearrangements from room temperature to 80 °C.
 Analogous to the rate acceleration observed in the anionic-oxy-
Analogous to the rate acceleration observed in the anionic-oxy-Cope rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields ...
Danheiser reported a very similar effect for vinylcyclopropane substrates bearing lkoxysubstituents.
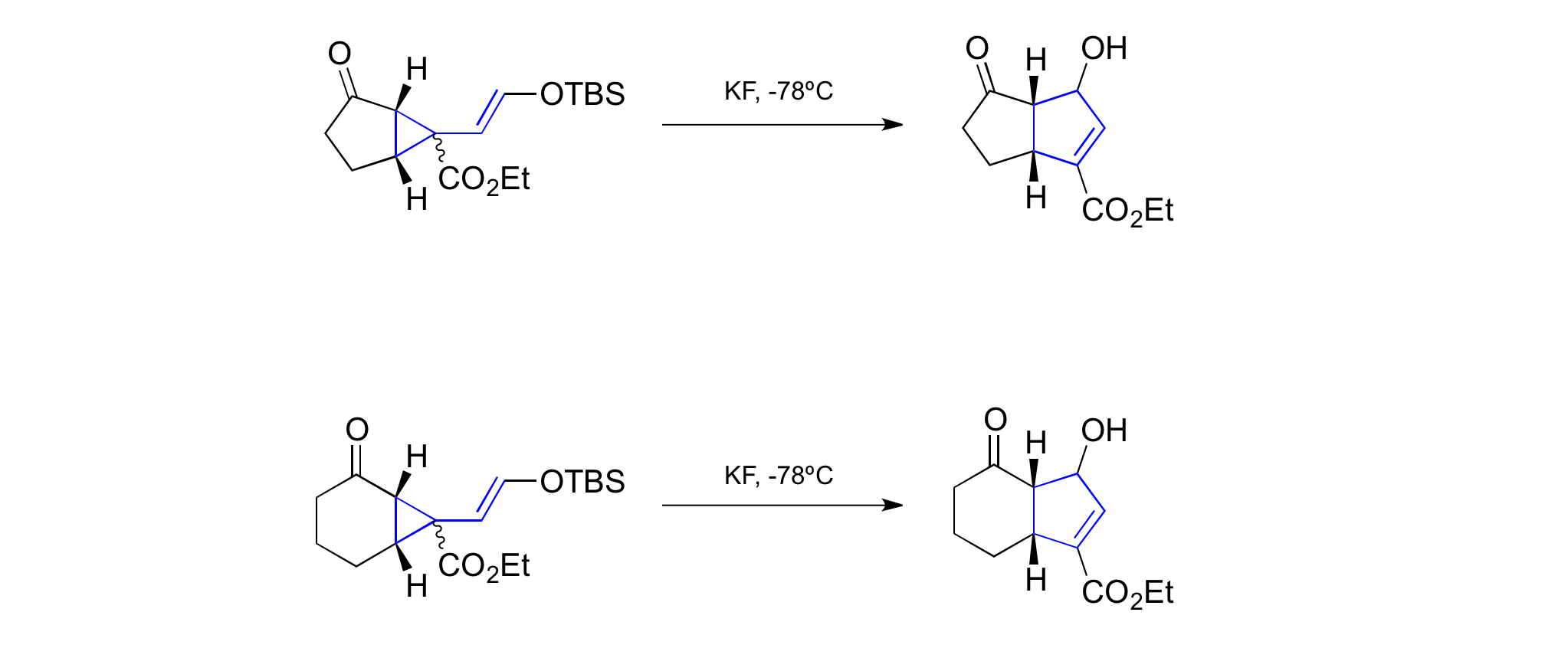
 Another intriguing result was reported by Larsen in 1988. He was able to promote vinylcyclopropane rearrangements with substrates such as the one shown in the reaction below at temperatures as low as -78 °C. The substrates were generated ''in situ'' upon ringcontracting thiocarbonyl Diels-Alder adducts under basic conditions. This methodology allowed the formation of numerous highly functionalized cyclopentenes in a
Another intriguing result was reported by Larsen in 1988. He was able to promote vinylcyclopropane rearrangements with substrates such as the one shown in the reaction below at temperatures as low as -78 °C. The substrates were generated ''in situ'' upon ringcontracting thiocarbonyl Diels-Alder adducts under basic conditions. This methodology allowed the formation of numerous highly functionalized cyclopentenes in a stereoselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
manner.
 Another low temperature vinylcyclopropane rearrangement was obtained by the Hudlicky group. The scope of this particular methodology is impressively broad and allows the formation of various -5 as well as -6carbon scaffolds.
Another low temperature vinylcyclopropane rearrangement was obtained by the Hudlicky group. The scope of this particular methodology is impressively broad and allows the formation of various -5 as well as -6carbon scaffolds.

Use in total synthesis
Five-membered carbon rings are ubiquitous structural motifs in natural products. In contrast to the larger, fully "consonant"cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
scaffold cyclopentanes and their derivatives are "dissonant
In music, consonance and dissonance are categorizations of simultaneous or successive Sound, sounds. Within the Western tradition, some listeners associate consonance with sweetness, pleasantness, and acceptability, and dissonance with harshness ...
" according to the Lapworth-Evans model of alternating polarities. The dissonance in polarity clearly limits the ways by which cyclopentanes can be disconnected which becomes evident in the decreased number of general methods available for making five-membered rings versus the corresponding six-membered rings. Especially the fact that there is no Diels-Alder-equivalent for the synthesis of five-membered rings has been bothering synthetic chemists for many decades. Consequentially, after the vinylcyclopropane rearrangement was discovered around 1960 it didn't take long for the synthetic community to realize the potential inherent to form cyclopentenes by means of the vinylcyclopropane rearrangement. As the vinylcyclopropane rearrangement progressed as a methodology and the reaction conditions improved during the 1970s, first total syntheses making use of the vinylcyclopropane rearrangement started to appear around 1980. Key figures to apply this reaction in total synthesis were Barry M. Trost, Elias J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many ...
, Thomas Hudlicky, Leo A. Paquette,
Trost's synthesis of aphidicolin (1979)
In 1979 Trost reported the synthesis ofAphidicolin
Aphidicolin is a tetracyclic diterpene antibiotic isolated from the fungus '' Cephalosporum aphidicola'' with antiviral and antimitotic properties. Aphidicolin is a reversible inhibitor of eukaryotic nuclear DNA replication. It blocks the cell ...
using methodology around the vinylcyclopropane rearrangement developed in their own laboratory . In one of their key steps they were able to convert a late stage siloxyvinylcyclopropane into a cyclopentene that contained the -6-5fused carbon skeleton found within the natural product. They were able to convert the rearranged product into the natural product by further manipulations.

Piers' synthesis of zizaene (1979)
Piers' synthesis of zizaene is another early example for the application of a vinylcyclopropane rearrangement as a key disconnection.
Hudlicky's synthesis of hirstuene (1980) and isocomene (1984)
Hudlicky has been one of the key figures in pushing the vinylcyclopropane rearrangements forwards as a method and has used in multiple times in complex natural product synthesis. A particularly elegant piece of work is the chemistry developed to access both, linear as well as angular triquinanes starting from similar precursors. He has been able to apply this strategy to hirsutene and isocomene
Paquette's synthesis of alpha-Vetispirene (1982)
Paquette used a vinylcyclopropane rearrangement to build the spirocyclic natural product alpha-Vetispirene in 1982.
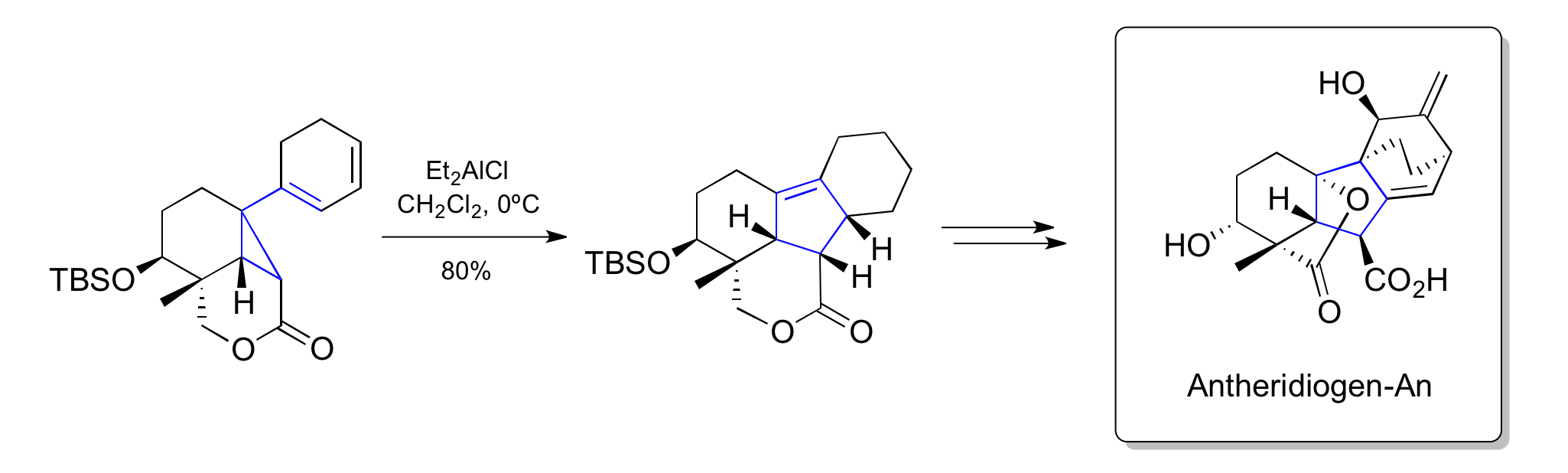
Corey's synthesis of Antheridiogen-An (1985)
Elias J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many ...
has contributed heavily to the development of the vinylcyclopropane rearrangement as a synthetic method. In 1985, Corey and his student, Andrew G. Myers, published an impressive synthesis of Antheridiogen-An using a Lewis-acid mediated late-stage vinylcyclopropane rearrangement.

Njardarson's synthesis of biotin (2007)
More recently a copper-catalyzed heteroatom-vinylcyclopropane rearrangement was used to form thetetrahydrothiophene
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorles ...
core of biotin
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', bor ...
and the thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
unit of Plavix
Clopidogrel — sold under the brand name Plavix, among others — is an antiplatelet drug, antiplatelet medication used to reduce the risk of Cardiovascular disease, heart disease and stroke in those at high risk. It is also used together wit ...
respectively.

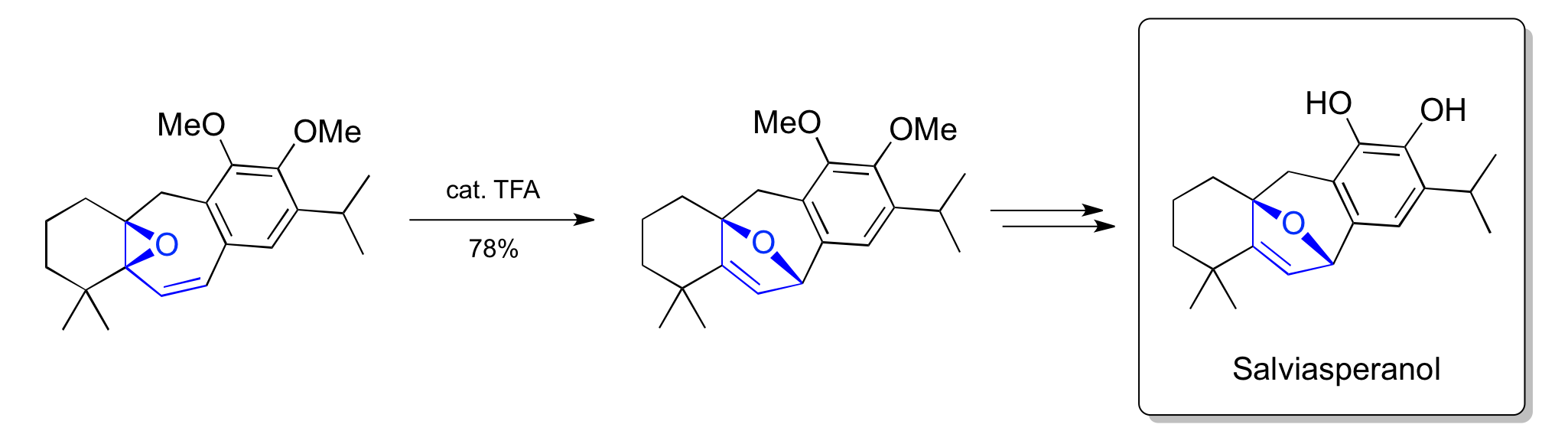
Majetich's's synthesis of salviasperanol (2008)
In 2008, an acid-mediated vinylcyclopropane rearrangement was used to synthesize the natural product salviasperanol.
See also
*Skattebøl rearrangement The Skattebøl rearrangement is an organic reaction for converting a geminal dihalo cyclopropane to an allene using an organolithium base. This rearrangement reaction is named after its discoverer, Lars Skattebøl, Professor emeritus at the Univer ...
* Di-π-methane rearrangement The di-π-methane rearrangement is a photochemical reaction of a molecular entity that contains two π-systems separated by a saturated carbon atom (a 1,4-diene or an allyl-substituted aromatic ring), to form an ene- (or aryl-) substituted cyclop ...
References
{{Reflist, 2 Rearrangement reactions Cyclopropanes