Thorium on:
[Wikipedia]
[Google]
[Amazon]
Thorium is a weakly
 In total, 32 radioisotopes have been characterised, which range in mass number from 207 to 238. After 232Th, the most stable of them (with respective half-lives) are 230Th (75,380 years), 229Th (7,340 years), 228Th (1.92 years), 234Th (24.10 days), and 227Th (18.68 days). All of these isotopes occur in nature as trace radioisotopes due to their presence in the decay chains of 232Th, 235U, 238U, and 237 Np: the last of these is long
In total, 32 radioisotopes have been characterised, which range in mass number from 207 to 238. After 232Th, the most stable of them (with respective half-lives) are 230Th (75,380 years), 229Th (7,340 years), 228Th (1.92 years), 234Th (24.10 days), and 227Th (18.68 days). All of these isotopes occur in nature as trace radioisotopes due to their presence in the decay chains of 232Th, 235U, 238U, and 237 Np: the last of these is long
 Despite the anomalous electron configuration for gaseous thorium atoms, metallic thorium shows significant 5f involvement. A hypothetical metallic state of thorium that had the nd27s2 configuration with the 5f orbitals above the
Despite the anomalous electron configuration for gaseous thorium atoms, metallic thorium shows significant 5f involvement. A hypothetical metallic state of thorium that had the nd27s2 configuration with the 5f orbitals above the


 In January 2021, the aromaticity has been observed in a large metal cluster anion consisting of 12 bismuth atoms stabilised by a center thorium cation. This compound was shown to be surprisingly stable, unlike many previous known aromatic metal clusters.
In January 2021, the aromaticity has been observed in a large metal cluster anion consisting of 12 bismuth atoms stabilised by a center thorium cation. This compound was shown to be surprisingly stable, unlike many previous known aromatic metal clusters.
 In the universe, thorium is among the rarest of the primordial elements, because it is one of the two elements that can be produced only in the r-process (the other being uranium), and also because it has slowly been decaying away from the moment it formed. The only primordial elements rarer than thorium are thulium, lutetium, tantalum, and rhenium, the odd-numbered elements just before the third peak of r-process abundances around the heavy platinum group metals, as well as uranium. In the distant past the abundances of thorium and uranium were enriched by the decay of plutonium and curium isotopes, and thorium was enriched relative to uranium by the decay of 236U to 232Th and the natural depletion of 235U, but these sources have long since decayed and no longer contribute.
In the Earth's crust, thorium is much more abundant: with an abundance of 8.1 g/
In the universe, thorium is among the rarest of the primordial elements, because it is one of the two elements that can be produced only in the r-process (the other being uranium), and also because it has slowly been decaying away from the moment it formed. The only primordial elements rarer than thorium are thulium, lutetium, tantalum, and rhenium, the odd-numbered elements just before the third peak of r-process abundances around the heavy platinum group metals, as well as uranium. In the distant past the abundances of thorium and uranium were enriched by the decay of plutonium and curium isotopes, and thorium was enriched relative to uranium by the decay of 236U to 232Th and the natural depletion of 235U, but these sources have long since decayed and no longer contribute.
In the Earth's crust, thorium is much more abundant: with an abundance of 8.1 g/
 Berzelius made some initial characterizations of the new metal and its chemical compounds: he correctly determined that the thorium–oxygen mass ratio of thorium oxide was 7.5 (its actual value is close to that, ~7.3), but he assumed the new element was divalent rather than tetravalent, and so calculated that the atomic mass was 7.5 times that of oxygen (120 amu); it is actually 15 times as large. He determined that thorium was a very electropositive metal, ahead of cerium and behind zirconium in electropositivity. Metallic thorium was isolated for the first time in 1914 by Dutch entrepreneurs Dirk Lely Jr. and Lodewijk Hamburger.
Berzelius made some initial characterizations of the new metal and its chemical compounds: he correctly determined that the thorium–oxygen mass ratio of thorium oxide was 7.5 (its actual value is close to that, ~7.3), but he assumed the new element was divalent rather than tetravalent, and so calculated that the atomic mass was 7.5 times that of oxygen (120 amu); it is actually 15 times as large. He determined that thorium was a very electropositive metal, ahead of cerium and behind zirconium in electropositivity. Metallic thorium was isolated for the first time in 1914 by Dutch entrepreneurs Dirk Lely Jr. and Lodewijk Hamburger.
 While thorium was discovered in 1828 its first application dates only from 1885, when Austrian chemist Carl Auer von Welsbach invented the gas mantle, a portable source of light which produces light from the incandescence of thorium oxide when heated by burning gaseous fuels. Many applications were subsequently found for thorium and its compounds, including ceramics, carbon arc lamps, heat-resistant crucibles, and as catalysts for industrial chemical reactions such as the oxidation of ammonia to nitric acid.
While thorium was discovered in 1828 its first application dates only from 1885, when Austrian chemist Carl Auer von Welsbach invented the gas mantle, a portable source of light which produces light from the incandescence of thorium oxide when heated by burning gaseous fuels. Many applications were subsequently found for thorium and its compounds, including ceramics, carbon arc lamps, heat-resistant crucibles, and as catalysts for industrial chemical reactions such as the oxidation of ammonia to nitric acid.

 Thorium has been used as a power source on a prototype scale. The earliest thorium-based reactor was built at the
Thorium has been used as a power source on a prototype scale. The earliest thorium-based reactor was built at the
radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
metallic chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Th and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
. Thorium is an electropositive actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
whose chemistry is dominated by the +4 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
; it is quite reactive and can ignite in air when finely divided.
All known thorium isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s are unstable. The most stable isotope, 232Th, has a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 14.05 billion years, or about the age of the universe; it decays very slowly via alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
, starting a decay chain named the thorium series that ends at stable 208 Pb. On Earth, thorium and uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
are the only significantly radioactive elements that still occur naturally in large quantities as primordial elements. Thorium is estimated to be over three times as abundant as uranium in the Earth's crust, and is chiefly refined from monazite sands as a by-product of extracting rare-earth metal
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silv ...
s.
Thorium was discovered in 1828 by the Norwegian amateur mineralogist Morten Thrane Esmark and identified by the Swedish chemist Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be o ...
, who named it after Thor
Thor (; from non, Þórr ) is a prominent god in Germanic paganism. In Norse mythology, he is a hammer-wielding god associated with lightning, thunder, storms, sacred groves and trees, strength, the protection of humankind, hallowing, ...
, the Norse god of thunder. Its first applications were developed in the late 19th century. Thorium's radioactivity was widely acknowledged during the first decades of the 20th century. In the second half of the century, thorium was replaced in many uses due to concerns about its radioactivity.
Thorium is still being used as an alloying element in TIG welding electrodes but is slowly being replaced in the field with different compositions. It was also material in high-end optics and scientific instrumentation, used in some broadcast vacuum tubes, and as the light source in gas mantles, but these uses have become marginal. It has been suggested as a replacement for uranium as nuclear fuel in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s, and several thorium reactors have been built. Thorium is also used in strengthening magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, coating tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
wire in electrical equipment, controlling the grain size of tungsten in electric lamps, high-temperature crucibles, and glasses including camera and scientific instrument lenses. Other uses for thorium include heat-resistant ceramics, aircraft engines
An aircraft engine, often referred to as an aero engine, is the power component of an aircraft propulsion system. Most aircraft engines are either piston engines or gas turbines, although a few have been rocket powered and in recent years man ...
, and in light bulbs. Ocean science has utilised 231 Pa/230Th isotope ratios to understand the ancient ocean.
Bulk properties
Thorium is a moderately soft, paramagnetic, bright silvery radioactive actinide metal. In theperiodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, it lies to the right of actinium
Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance ...
, to the left of protactinium, and below cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
. Pure thorium is very ductile and, as normal for metals, can be cold-rolled, swaged, and drawn. At room temperature, thorium metal has a face-centred cubic crystal structure; it has two other forms, one at high temperature (over 1360 °C; body-centred cubic) and one at high pressure (around 100 GPa; body-centred tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a squar ...
).
Thorium metal has a bulk modulus
The bulk modulus (K or B) of a substance is a measure of how resistant to compression the substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume.
Other moduli descri ...
(a measure of resistance to compression of a material) of 54 GPa, about the same as tin's (58.2 GPa). Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
's is 75.2 GPa; copper's 137.8 GPa; and mild steel's is 160–169 GPa. Thorium is about as hard as soft steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
, so when heated it can be rolled into sheets and pulled into wire.
Thorium is nearly half as dense as uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
and is harder than both. It becomes superconductive below 1.4 K. Thorium's melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
of 1750 °C is above both those of actinium (1227 °C) and protactinium (1568 °C). At the start of period 7
A period 7 element is one of the chemical elements in the seventh row (or ''period'') of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of th ...
, from francium to thorium, the melting points of the elements increase (as in other periods), because the number of delocalised electrons each atom contributes increases from one in francium to four in thorium, leading to greater attraction between these electrons and the metal ions as their charge increases from one to four. After thorium, there is a new downward trend in melting points from thorium to plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
, where the number of f electrons increases from about 0.4 to about 6: this trend is due to the increasing hybridisation of the 5f and 6d orbitals and the formation of directional bonds resulting in more complex crystal structures and weakened metallic bonding. (The f-electron count for thorium metal is a non-integer due to a 5f–6d overlap.) Among the actinides up to californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding c ...
, which can be studied in at least milligram quantities, thorium has the highest melting and boiling points and second-lowest density; only actinium is lighter. Thorium's boiling point of 4788 °C is the fifth-highest among all the elements with known boiling points.
The properties of thorium vary widely depending on the degree of impurities in the sample. The major impurity is usually thorium dioxide ); even the purest thorium specimens usually contain about a tenth of a percent of the dioxide. Experimental measurements of its density give values between 11.5 and 11.66 g/cm3: these are slightly lower than the theoretically expected value of 11.7 g/cm3 calculated from thorium's lattice parameters, perhaps due to microscopic voids forming in the metal when it is cast. These values lie between those of its neighbours actinium (10.1 g/cm3) and protactinium (15.4 g/cm3), part of a trend across the early actinides.
Thorium can form alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
s with many other metals. Addition of small proportions of thorium improves the mechanical strength of magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, and thorium-aluminum alloys have been considered as a way to store thorium in proposed future thorium nuclear reactors. Thorium forms eutectic mixtures with chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
and uranium, and it is completely miscible in both solid and liquid states with its lighter congener cerium.
Isotopes
All but two elements up to bismuth (element 83) have an isotope that is practically stable for all purposes ("classically stable"), with the exceptions being technetium andpromethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
(elements 43 and 61). All elements from polonium (element 84) onward are measurably radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
. 232Th is one of the two nuclides beyond bismuth (the other being 238U) that have half-lives measured in billions of years; its half-life is 14.05 billion years, about three times the age of the earth, and slightly longer than the age of the universe. Four-fifths of the thorium present at Earth's formation has survived to the present. 232Th is the only isotope of thorium occurring in quantity in nature. Its stability is attributed to its closed nuclear subshell with 142 neutrons. Thorium has a characteristic terrestrial isotopic composition, with atomic weight 232.0377(4). It is one of only four radioactive elements (along with bismuth, protactinium and uranium) that occur in large enough quantities on Earth for a standard atomic weight to be determined.
Thorium nuclei are susceptible to alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
because the strong nuclear force cannot overcome the electromagnetic repulsion between their protons. The alpha decay of 232Th initiates the 4''n'' decay chain which includes isotopes with a mass number divisible by 4 (hence the name; it is also called the thorium series after its progenitor). This chain of consecutive alpha and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
s begins with the decay of 232Th to 228Ra and terminates at 208Pb. Any sample of thorium or its compounds contains traces of these daughters, which are isotopes of thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
, lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
, bismuth, polonium, radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains th ...
, radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rat ...
, and actinium. Natural thorium samples can be chemically purified to extract useful daughter nuclides, such as 212Pb, which is used in nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
for cancer therapy
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
. 227Th (alpha emitter with an 18.68 days half-life) can also be used in cancer treatments such as targeted alpha therapies. 232Th also very occasionally undergoes spontaneous fission rather than alpha decay, and has left evidence of doing so in its minerals (as trapped xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
gas formed as a fission product), but the partial half-life of this process is very large at over 1021 years and alpha decay predominates.
extinct
Extinction is the termination of a kind of organism or of a group of kinds (taxon), usually a species. The moment of extinction is generally considered to be the death of the last individual of the species, although the capacity to breed and ...
in nature due to its short half-life (2.14 million years), but is continually produced in minute traces from neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
in uranium ores. All of the remaining thorium isotopes have half-lives that are less than thirty days and the majority of these have half-lives that are less than ten minutes.
In deep seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
s the isotope 230Th makes up to 0.04% of natural thorium. This is because its parent 238U is soluble in water, but 230Th is insoluble and precipitates into the sediment. Uranium ores with low thorium concentrations can be purified to produce gram-sized thorium samples of which over a quarter is the 230Th isotope, since 230Th is one of the daughters of 238U. The International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) reclassified thorium as a binuclidic element in 2013; it had formerly been considered a mononuclidic element.
Thorium has three known nuclear isomers (or metastable states), 216m1Th, 216m2Th, and 229mTh. 229mTh has the lowest known excitation energy of any isomer, measured to be . This is so low that when it undergoes isomeric transition, the emitted gamma radiation is in the ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation ...
range.
Different isotopes of thorium are chemically identical, but have slightly differing physical properties: for example, the densities of pure 228Th, 229Th, 230Th, and 232Th are respectively expected to be 11.5, 11.6, 11.6, and 11.7 g/cm3. The isotope 229Th is expected to be fissionable with a bare critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fi ...
of 2839 kg, although with steel reflectors this value could drop to 994 kg. 232Th is not fissionable, but it is fertile as it can be converted to fissile 233U by neutron capture and subsequent beta decay.
Radiometric dating
Two radiometric dating methods involve thorium isotopes: uranium–thorium dating, based on the decay of 234U to 230Th, and ionium–thorium dating, which measures the ratio of 232Th to 230Th. These rely on the fact that 232Th is a primordial radioisotope, but 230Th only occurs as an intermediate decay product in the decay chain of 238U. Uranium–thorium dating is a relatively short-range process because of the short half-lives of 234U and 230Th relative to the age of the Earth: it is also accompanied by a sister process involving the alpha decay of 235U into 231Th, which very quickly becomes the longer-lived 231Pa, and this process is often used to check the results of uranium–thorium dating. Uranium–thorium dating is commonly used to determine the age of calcium carbonate materials such asspeleothem
A speleothem (; ) is a geological formation by mineral deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, dependi ...
or coral
Corals are marine invertebrates within the class Anthozoa of the phylum Cnidaria. They typically form compact colonies of many identical individual polyps. Coral species include the important reef builders that inhabit tropical oceans and se ...
, because uranium is more soluble in water than thorium and protactinium, which are selectively precipitated into ocean-floor sediment
Sediment is a naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of wind, water, or ice or by the force of gravity acting on the particles. For example, sand ...
s, where their ratios are measured. The scheme has a range of several hundred thousand years. Ionium–thorium dating is a related process, which exploits the insolubility of thorium (both 232Th and 230Th) and thus its presence in ocean sediments to date these sediments by measuring the ratio of 232Th to 230Th. Both of these dating methods assume that the proportion of 230Th to 232Th is a constant during the period when the sediment layer was formed, that the sediment did not already contain thorium before contributions from the decay of uranium, and that the thorium cannot migrate within the sediment layer.
Chemistry
A thorium atom has 90 electrons, of which four are valence electrons. Fouratomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any ...
s are theoretically available for the valence electrons to occupy: 5f, 6d, 7s, and 7p. Despite thorium's position in the f-block of the periodic table, it has an anomalous nd27s2 electron configuration in the ground state, as the 5f and 6d subshells in the early actinides are very close in energy, even more so than the 4f and 5d subshells of the lanthanides: thorium's 6d subshells are lower in energy than its 5f subshells, because its 5f subshells are not well-shielded by the filled 6s and 6p subshells and are destabilized. This is due to relativistic effects
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of ...
, which become stronger near the bottom of the periodic table, specifically the relativistic spin–orbit interaction
In quantum physics, the spin–orbit interaction (also called spin–orbit effect or spin–orbit coupling) is a relativistic interaction of a particle's spin with its motion inside a potential. A key example of this phenomenon is the spin–orb ...
. The closeness in energy levels of the 5f, 6d, and 7s energy levels of thorium results in thorium almost always losing all four valence electrons and occurring in its highest possible oxidation state of +4. This is different from its lanthanide congener cerium, in which +4 is also the highest possible state, but +3 plays an important role and is more stable. Thorium is much more similar to the transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s zirconium and hafnium than to cerium in its ionization energies and redox potentials, and hence also in its chemistry: this transition-metal-like behaviour is the norm in the first half of the actinide series.
 Despite the anomalous electron configuration for gaseous thorium atoms, metallic thorium shows significant 5f involvement. A hypothetical metallic state of thorium that had the nd27s2 configuration with the 5f orbitals above the
Despite the anomalous electron configuration for gaseous thorium atoms, metallic thorium shows significant 5f involvement. A hypothetical metallic state of thorium that had the nd27s2 configuration with the 5f orbitals above the Fermi level
The Fermi level of a solid-state body is the thermodynamic work required to add one electron to the body. It is a thermodynamic quantity usually denoted by ''µ'' or ''E''F
for brevity. The Fermi level does not include the work required to remove ...
should be hexagonal close packed like the group 4 element
Group 4 is the second group of transition metals in the periodic table. It contains the four elements titanium (Ti), zirconium (Zr), hafnium (Hf), and rutherfordium (Rf). The group is also called the titanium group or titanium family after its lig ...
s titanium, zirconium, and hafnium, and not face-centred cubic as it actually is. The actual crystal structure can only be explained when the 5f states are invoked, proving that thorium is metallurgically a true actinide.
Tetravalent thorium compounds are usually colourless or yellow, like those of silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
or lead, as the ion has no 5f or 6d electrons. Thorium chemistry is therefore largely that of an electropositive metal forming a single diamagnetic ion with a stable noble-gas configuration, indicating a similarity between thorium and the main group element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arran ...
s of the s-block. Thorium and uranium are the most investigated of the radioactive elements because their radioactivity is low enough not to require special handling in the laboratory.
Reactivity
Thorium is a highly reactive and electropositive metal. With a standard reduction potential of −1.90 V for the /Th couple, it is somewhat more electropositive than zirconium or aluminium. Finely divided thorium metal can exhibit pyrophoricity, spontaneously igniting in air. When heated in air, thorium turnings ignite and burn with a brilliant white light to produce the dioxide. In bulk, the reaction of pure thorium with air is slow, although corrosion may occur after several months; most thorium samples are contaminated with varying degrees of the dioxide, which greatly accelerates corrosion. Such samples slowly tarnish, becoming grey and finally black at the surface. At standard temperature and pressure, thorium is slowly attacked by water, but does not readily dissolve in most common acids, with the exception ofhydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dige ...
, where it dissolves leaving a black insoluble residue of ThO(OH,Cl)H. It dissolves in concentrated nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
containing a small quantity of catalytic fluoride or fluorosilicate ions; if these are not present, passivation by the nitrate can occur, as with uranium and plutonium.

Inorganic compounds
Most binary compounds of thorium with nonmetals may be prepared by heating the elements together. In air, thorium burns to form , which has the fluorite structure. Thorium dioxide is arefractory material
In materials science, a refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, ...
, with the highest melting point (3390 °C) of any known oxide. It is somewhat hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance' ...
and reacts readily with water and many gases; it dissolves easily in concentrated nitric acid in the presence of fluoride.
When heated in air, thorium dioxide emits intense blue light; the light becomes white when is mixed with its lighter homologue cerium dioxide (, ceria): this is the basis for its previously common application in gas mantles. A flame is not necessary for this effect: in 1901, it was discovered that a hot Welsbach gas mantle (using with 1% ) remained at "full glow" when exposed to a cold unignited mixture of flammable gas and air. The light emitted by thorium dioxide is higher in wavelength than the blackbody emission expected from incandescence
Incandescence is the emission of electromagnetic radiation (including visible light) from a hot body as a result of its high temperature. The term derives from the Latin verb ''incandescere,'' to glow white. A common use of incandescence is ...
at the same temperature, an effect called candoluminescence Candoluminescence is the light given off by certain materials at elevated temperatures (usually when exposed to a flame) that has an intensity at some wavelengths which can, through chemical action in flames, be higher than the blackbody emission ex ...
. It occurs because : Ce acts as a catalyst for the recombination of free radicals that appear in high concentration in a flame, whose deexcitation releases large amounts of energy. The addition of 1% cerium dioxide, as in gas mantles, heightens the effect by increasing emissivity in the visible region of the spectrum; but because cerium, unlike thorium, can occur in multiple oxidation states, its charge and hence visible emissivity will depend on the region on the flame it is found in (as such regions vary in their chemical composition and hence how oxidising or reducing they are).
Several binary thorium chalcogenides and oxychalcogenides are also known with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
, selenium, and tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionall ...
.
All four thorium tetrahalides are known, as are some low-valent bromides and iodides: the tetrahalides are all 8-coordinated hygroscopic compounds that dissolve easily in polar solvents such as water. Many related polyhalide ions are also known. Thorium tetrafluoride has a monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic ...
crystal structure like those of zirconium tetrafluoride and hafnium tetrafluoride, where the ions are coordinated with ions in somewhat distorted square antiprisms. The other tetrahalides instead have dodecahedral geometry. Lower iodides (black) and (gold-coloured) can also be prepared by reducing the tetraiodide with thorium metal: they do not contain Th(III) and Th(II), but instead contain and could be more clearly formulated as electride compounds. Many polynary halides with the alkali metals, barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
, thallium, and ammonium are known for thorium fluorides, chlorides, and bromides. For example, when treated with potassium fluoride and hydrofluoric acid, forms the complex anion (hexafluorothorate(IV)), which precipitates as an insoluble salt, (potassium hexafluorothorate(IV)).
Thorium borides, carbides, silicides, and nitrides are refractory materials, like those of uranium and plutonium, and have thus received attention as possible nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergo ...
s. All four heavier pnictogens (phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, b ...
, antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient ti ...
, and bismuth) also form binary thorium compounds. Thorium germanides are also known. Thorium reacts with hydrogen to form the thorium hydrides and , the latter of which is superconducting below 7.5–8 K; at standard temperature and pressure, it conducts electricity like a metal. The hydrides are thermally unstable and readily decompose upon exposure to air or moisture.

Coordination compounds
In an acidic aqueous solution, thorium occurs as the tetrapositive aqua ion , which hastricapped trigonal prismatic molecular geometry
In chemistry, the tricapped trigonal prismatic molecular geometry describes the shape of compounds where nine atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a triaugmented triangular prism (a trigo ...
: at pH < 3, the solutions of thorium salts are dominated by this cation. The ion is the largest of the tetrapositive actinide ions, and depending on the coordination number can have a radius between 0.95 and 1.14 Å. It is quite acidic due to its high charge, slightly stronger than sulfurous acid
Sulfurous acid (also sulfuric(IV) acid, sulphurous acid (UK), sulphuric(IV) acid (UK)) is the chemical compound with the formula . There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase ...
: thus it tends to undergo hydrolysis and polymerisation (though to a lesser extent than ), predominantly to in solutions with pH 3 or below, but in more alkaline solution polymerisation continues until the gelatinous hydroxide forms and precipitates out (though equilibrium may take weeks to be reached, because the polymerisation usually slows down before the precipitation). As a hard Lewis acid, favours hard ligands with oxygen atoms as donors: complexes with sulfur atoms as donors are less stable and are more prone to hydrolysis.
High coordination numbers are the rule for thorium due to its large size. Thorium nitrate pentahydrate was the first known example of coordination number 11, the oxalate tetrahydrate has coordination number 10, and the borohydride (first prepared in the Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
) has coordination number 14. These thorium salts are known for their high solubility in water and polar organic solvents.
Many other inorganic thorium compounds with polyatomic anions are known, such as the perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, . The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. ...
s, sulfates, sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are w ...
s, nitrates, carbonates, phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
s, vanadates, molybdate
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, altho ...
s, and chromates
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ...
, and their hydrated forms. They are important in thorium purification and the disposal of nuclear waste, but most of them have not yet been fully characterized, especially regarding their structural properties. For example, thorium nitrate is produced by reacting thorium hydroxide with nitric acid: it is soluble in water and alcohols and is an important intermediate in the purification of thorium and its compounds. Thorium complexes with organic ligands, such as oxalate, citrate, and EDTA, are much more stable. In natural thorium-containing waters, organic thorium complexes usually occur in concentrations orders of magnitude higher than the inorganic complexes, even when the concentrations of inorganic ligands are much greater than those of organic ligands.
Organothorium compounds
Most of the work on organothorium compounds has focused on thecyclopentadienyl complex
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadien ...
es and cyclooctatetraenyls. Like many of the early and middle actinides (up to americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was n ...
, and also expected for curium), thorium forms a cyclooctatetraenide complex: the yellow , thorocene
Actinocenes are a family of organoactinide compounds consisting of metallocenes containing elements from the actinide series. They typically have a sandwich structure with two dianionic cyclooctatetraenyl ligands (COT2-, which is ) bound to an a ...
. It is isotypic with the better-known analogous uranium compound uranocene. It can be prepared by reacting with thorium tetrachloride in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF) at the temperature of dry ice, or by reacting thorium tetrafluoride with . It is unstable in air and decomposes in water or at 190 °C. Half sandwich compounds are also known, such as , which has a piano-stool structure and is made by reacting thorocene with thorium tetrachloride in tetrahydrofuran.
The simplest of the cyclopentadienyls are and : many derivatives are known. The former (which has two forms, one purple and one green) is a rare example of thorium in the formal +3 oxidation state; a formal +2 oxidation state occurs in a derivative. The chloride derivative is prepared by heating thorium tetrachloride with limiting used (other univalent metal cyclopentadienyls can also be used). The alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
derivatives are prepared from the chloride derivative and have been used to study the nature of the Th–C sigma bond.
Other organothorium compounds are not well-studied. Tetrabenzylthorium, , and tetraallylthorium, , are known, but their structures have not been determined. They decompose slowly at room temperature. Thorium forms the monocapped trigonal prismatic anion , heptamethylthorate(IV), which forms the salt (tmeda = ). Although one methyl group is only attached to the thorium atom (Th–C distance 257.1 pm) and the other six connect the lithium and thorium atoms (Th–C distances 265.5–276.5 pm), they behave equivalently in solution. Tetramethylthorium, , is not known, but its adducts are stabilised by phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands.
Occurrence
Formation
232Th is a primordial nuclide, having existed in its current form for over ten billion years; it was formed during the r-process, which probably occurs insupernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or whe ...
e and neutron star mergers. These violent events scattered it across the galaxy. The letter "r" stands for "rapid neutron capture", and occurs in core-collapse supernovae, where heavy seed nuclei such as 56Fe rapidly capture neutrons, running up against the neutron drip line
The nuclear drip line is the boundary beyond which atomic nuclei decay by the emission of a proton or neutron.
An arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus. One can think of moving up and/or to ...
, as neutrons are captured much faster than the resulting nuclides can beta decay back toward stability. Neutron capture is the only way for stars to synthesise elements beyond iron because of the increased Coulomb barriers that make interactions between charged particles difficult at high atomic numbers and the fact that fusion beyond 56Fe is endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. ...
. Because of the abrupt loss of stability past 209Bi, the r-process is the only process of stellar nucleosynthesis that can create thorium and uranium; all other processes are too slow and the intermediate nuclei alpha decay before they capture enough neutrons to reach these elements.
tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
, it is one of the most abundant of the heavy elements, almost as abundant as lead (13 g/tonne) and more abundant than tin (2.1 g/tonne). This is because thorium is likely to form oxide minerals that do not sink into the core; it is classified as a lithophile under the Goldschmidt classification
The Goldschmidt classification,
developed by Victor Goldschmidt (1888–1947), is a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile ...
, meaning that it is generally found combined with oxygen. Common thorium compounds are also poorly soluble in water. Thus, even though the refractory elements have the same relative abundances in the Earth as in the Solar System as a whole, there is more accessible thorium than heavy platinum group metals in the crust.
On Earth
Thorium is the 41st most abundant element in the Earth's crust. Natural thorium is usually almost pure 232Th, which is the longest-lived and most stable isotope of thorium, having a half-life comparable to the age of the universe. Its radioactive decay is the largest single contributor to the Earth's internal heat; the other major contributors are the shorter-lived primordial radionuclides, which are 238U, 40K, and 235U in descending order of their contribution. (At the time of the Earth's formation, 40K and 235U contributed much more by virtue of their short half-lives, but they have decayed more quickly, leaving the contribution from 232Th and 238U predominant.) Its decay accounts for a gradual decrease of thorium content of the Earth: the planet currently has around 85% of the amount present at the formation of the Earth. The other natural thorium isotopes are much shorter-lived; of them, only 230Th is usually detectable, occurring in secular equilibrium with its parent 238U, and making up at most 0.04% of natural thorium. Thorium only occurs as a minor constituent of most minerals, and was for this reason previously thought to be rare. In nature, thorium occurs in the +4 oxidation state, together with uranium(IV), zirconium(IV), hafnium(IV), and cerium(IV), and also withscandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
, yttrium, and the trivalent lanthanides which have similar ionic radii
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation ...
. Because of thorium's radioactivity, minerals containing it are often metamict (amorphous), their crystal structure having been damaged by the alpha radiation produced by thorium. An extreme example is ekanite, , which almost never occurs in nonmetamict form due to the thorium it contains.
Monazite (chiefly phosphates of various rare-earth elements) is the most important commercial source of thorium because it occurs in large deposits worldwide, principally in India, South Africa, Brazil, Australia, and Malaysia
Malaysia ( ; ) is a country in Southeast Asia. The federal constitutional monarchy consists of thirteen states and three federal territories, separated by the South China Sea into two regions: Peninsular Malaysia and Borneo's East Mal ...
. It contains around 2.5% thorium on average, although some deposits may contain up to 20%. Monazite is a chemically unreactive mineral that is found as yellow or brown sand; its low reactivity makes it difficult to extract thorium from it. Allanite
Allanite (also called orthite) is a sorosilicate group of minerals within the broader epidote group that contain a significant amount of rare-earth elements. The mineral occurs mainly in metamorphosed clay-rich sediments and felsic igneous rocks. ...
(chiefly silicates-hydroxides of various metals) can have 0.1–2% thorium and zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of t ...
(chiefly zirconium silicate, ) up to 0.4% thorium.
Thorium dioxide occurs as the rare mineral thorianite
Thorianite is a rare thorium oxide mineral, ThO2. It was originally described by Ananda Coomaraswamy in 1904 as uraninite, but recognized as a new species by Wyndham R. Dunstan. It was so named by Dunstan on account of its high percentage of th ...
. Due to its being isotypic with uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear re ...
, these two common actinide dioxides can form solid-state solutions and the name of the mineral changes according to the content. Thorite (chiefly thorium silicate, ), also has a high thorium content and is the mineral in which thorium was first discovered. In thorium silicate minerals, the and ions are often replaced with (where M = Sc, Y, or Ln) and phosphate () ions respectively. Because of the great insolubility of thorium dioxide, thorium does not usually spread quickly through the environment when released. The ion is soluble, especially in acidic soils, and in such conditions the thorium concentration can be higher.
History
Erroneous report
In 1815, the Swedish chemistJöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be o ...
analysed an unusual sample of gadolinite
Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula . It is called gadolinite-(Ce) or gadolinite-(Y), depending o ...
from a copper mine in Falun, central Sweden. He noted impregnated traces of a white mineral, which he cautiously assumed to be an earth ( oxide in modern chemical nomenclature) of an unknown element. Berzelius had already discovered two elements, cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
and selenium, but he had made a public mistake once, announcing a new element, ''gahnium'', that turned out to be zinc oxide. Berzelius privately named the putative element "thorium" in 1817 and its supposed oxide "thorina" after Thor
Thor (; from non, Þórr ) is a prominent god in Germanic paganism. In Norse mythology, he is a hammer-wielding god associated with lightning, thunder, storms, sacred groves and trees, strength, the protection of humankind, hallowing, ...
, the Norse god of thunder. In 1824, after more deposits of the same mineral in Vest-Agder
Vest-Agder (; "West Agder") was one of 18 counties (''fylker'') in Norway up until 1 January 2020, when it was merged with Aust-Agder to form Agder county. In 2016, there were 182,701 inhabitants, around 3.5% of the total population of Norway. ...
, Norway, were discovered, he retracted his findings, as the mineral (later named xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as sili ...
) proved to be mostly yttrium orthophosphate.
Discovery
In 1828, Morten Thrane Esmark found a black mineral on Løvøya island, Telemark county, Norway. He was a Norwegianpriest
A priest is a religious leader authorized to perform the sacred rituals of a religion, especially as a mediatory agent between humans and one or more deities. They also have the authority or power to administer religious rites; in partic ...
and amateur mineralogist who studied the minerals in Telemark, where he served as vicar
A vicar (; Latin: '' vicarius'') is a representative, deputy or substitute; anyone acting "in the person of" or agent for a superior (compare "vicarious" in the sense of "at second hand"). Linguistically, ''vicar'' is cognate with the English pre ...
. He commonly sent the most interesting specimens, such as this one, to his father, Jens Esmark, a noted mineralogist and professor of mineralogy and geology at the Royal Frederick University in Christiania (today called Oslo
Oslo ( , , or ; sma, Oslove) is the capital and most populous city of Norway. It constitutes both a county and a municipality. The municipality of Oslo had a population of in 2022, while the city's greater urban area had a population of ...
). The elder Esmark determined that it was not a known mineral and sent a sample to Berzelius for examination. Berzelius determined that it contained a new element. He published his findings in 1829, having isolated an impure sample by reducing (potassium pentafluorothorate(IV)) with potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmos ...
metal. Berzelius reused the name of the previous supposed element discovery and named the source mineral thorite.
 Berzelius made some initial characterizations of the new metal and its chemical compounds: he correctly determined that the thorium–oxygen mass ratio of thorium oxide was 7.5 (its actual value is close to that, ~7.3), but he assumed the new element was divalent rather than tetravalent, and so calculated that the atomic mass was 7.5 times that of oxygen (120 amu); it is actually 15 times as large. He determined that thorium was a very electropositive metal, ahead of cerium and behind zirconium in electropositivity. Metallic thorium was isolated for the first time in 1914 by Dutch entrepreneurs Dirk Lely Jr. and Lodewijk Hamburger.
Berzelius made some initial characterizations of the new metal and its chemical compounds: he correctly determined that the thorium–oxygen mass ratio of thorium oxide was 7.5 (its actual value is close to that, ~7.3), but he assumed the new element was divalent rather than tetravalent, and so calculated that the atomic mass was 7.5 times that of oxygen (120 amu); it is actually 15 times as large. He determined that thorium was a very electropositive metal, ahead of cerium and behind zirconium in electropositivity. Metallic thorium was isolated for the first time in 1914 by Dutch entrepreneurs Dirk Lely Jr. and Lodewijk Hamburger.
Initial chemical classification
In the periodic table published byDmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
in 1869, thorium and the rare-earth elements were placed outside the main body of the table, at the end of each vertical period after the alkaline earth metals. This reflected the belief at that time that thorium and the rare-earth metals were divalent. With the later recognition that the rare earths were mostly trivalent and thorium was tetravalent, Mendeleev moved cerium and thorium to group IV in 1871, which also contained the modern carbon group (group 14) and titanium group (group 4), because their maximum oxidation state was +4. Cerium was soon removed from the main body of the table and placed in a separate lanthanide series; thorium was left with group 4 as it had similar properties to its supposed lighter congeners in that group, such as titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
and zirconium.
First uses
Radioactivity
Thorium was first observed to be radioactive in 1898, by the German chemist Gerhard Carl Schmidt and later that year, independently, by the Polish-French physicistMarie Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the fir ...
. It was the second element that was found to be radioactive, after the 1896 discovery of radioactivity in uranium by French physicist Henri Becquerel. Starting from 1899, the New Zealand physicist Ernest Rutherford and the American electrical engineer Robert Bowie Owens studied the radiation from thorium; initial observations showed that it varied significantly. It was determined that these variations came from a short-lived gaseous daughter of thorium, which they found to be a new element. This element is now named radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains th ...
, the only one of the rare radioelements to be discovered in nature as a daughter of thorium rather than uranium.
After accounting for the contribution of radon, Rutherford, now working with the British physicist Frederick Soddy, showed how thorium decayed at a fixed rate over time into a series of other elements in work dating from 1900 to 1903. This observation led to the identification of the half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
as one of the outcomes of the alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
experiments that led to the disintegration theory of radioactivity. The biological effect of radiation was discovered in 1903. The newly discovered phenomenon of radioactivity excited scientists and the general public alike. In the 1920s, thorium's radioactivity was promoted as a cure for rheumatism, diabetes
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ...
, and sexual impotence. In 1932, most of these uses were banned in the United States after a federal investigation into the health effects of radioactivity. 10,000 individuals in the United States had been injected with thorium during X-ray diagnosis; they were later found to suffer health issues such as leukaemia and abnormal chromosomes. Public interest in radioactivity had declined by the end of the 1930s.

Further classification
Up to the late 19th century, chemists unanimously agreed that thorium and uranium were the heaviest members of group 4 and group 6 respectively; the existence of the lanthanides in the sixth row was considered to be a one-off fluke. In 1892, British chemist Henry Bassett postulated a second extra-long periodic table row to accommodate known and undiscovered elements, considering thorium and uranium to be analogous to the lanthanides. In 1913, Danish physicist Niels Bohr published atheoretical model
A theory is a rational type of abstract thinking about a phenomenon, or the results of such thinking. The process of contemplative and rational thinking is often associated with such processes as observational study or research. Theories may be s ...
of the atom and its electron orbitals, which soon gathered wide acceptance. The model indicated that the seventh row of the periodic table should also have f-shells filling before the d-shells that were filled in the transition elements, like the sixth row with the lanthanides preceding the 5d transition metals. The existence of a second inner transition series, in the form of the actinides, was not accepted until similarities with the electron structures of the lanthanides had been established; Bohr suggested that the filling of the 5f orbitals may be delayed to after uranium.
It was only with the discovery of the first transuranic elements, which from plutonium onward have dominant +3 and +4 oxidation states like the lanthanides, that it was realised that the actinides were indeed filling f-orbitals rather than d-orbitals, with the transition-metal-like chemistry of the early actinides being the exception and not the rule. In 1945, when American physicist Glenn T. Seaborg and his team had discovered the transuranic elements americium and curium, he proposed the actinide concept
In nuclear chemistry, the actinide concept proposed that the actinides form a second inner transition series homologous to the lanthanides. Its origins stem from observation of lanthanide-like properties in transuranic elements in contrast to the d ...
, realising that thorium was the second member of an f-block actinide series analogous to the lanthanides, instead of being the heavier congener of hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
in a fourth d-block row.
Phasing out
In the 1990s, most applications that do not depend on thorium's radioactivity declined quickly due to safety and environmental concerns as suitable safer replacements were found. Despite its radioactivity, the element has remained in use for applications where no suitable alternatives could be found. A 1981 study by theOak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research an ...
in the United States estimated that using a thorium gas mantle every weekend would be safe for a person, but this was not the case for the dose received by people manufacturing the mantles or for the soils around some factory sites. Some manufacturers have changed to other materials, such as yttrium. As recently as 2007, some companies continued to manufacture and sell thorium mantles without giving adequate information about their radioactivity, with some even falsely claiming them to be non-radioactive.
Nuclear power
 Thorium has been used as a power source on a prototype scale. The earliest thorium-based reactor was built at the
Thorium has been used as a power source on a prototype scale. The earliest thorium-based reactor was built at the Indian Point Energy Center
Indian Point Energy Center (I.P.E.C.) is a three-unit nuclear power plant station located in Buchanan, just south of Peekskill, in Westchester County, New York. It sits on the east bank of the Hudson River, about north of Midtown Manhattan. T ...
located in Buchanan Buchanan may refer to:
People
* Buchanan (surname)
Places Africa
* Buchanan, Liberia, a large coastal town
Antarctica
* Buchanan Point, Laurie Island
Australia
* Buchanan, New South Wales
* Buchanan, Northern Territory, a locality
* Bucha ...
, New York, United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country Continental United States, primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., ...
in 1962. China may be the first to have a shot at commercializing the technology. The country with the largest estimated reserves of thorium in the world is India
India, officially the Republic of India (Hindi: ), is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area, the List of countries and dependencies by population, second-most populous ...
, which has sparse reserves of uranium. In the 1950s, India targeted achieving energy independence with their three-stage nuclear power programme. In most countries, uranium was relatively abundant and the progress of thorium-based reactors was slow; in the 20th century, three reactors were built in India and twelve elsewhere. Large-scale research was begun in 1996 by the International Atomic Energy Agency to study the use of thorium reactors; a year later, the United States Department of Energy
The United States Department of Energy (DOE) is an executive department of the U.S. federal government that oversees U.S. national energy policy and manages the research and development of nuclear power and nuclear weapons in the United States ...
started their research. Alvin Radkowsky Alvin Radkowsky (30 June 1915 – 17 February 2002) was an American nuclear physicist and chief scientist at U.S. Navy nuclear propulsion division. His work in the 1950s led to major advances in nuclear-ship technology and civilian use of nuclear po ...
of Tel Aviv University
Tel Aviv University (TAU) ( he, אוּנִיבֶרְסִיטַת תֵּל אָבִיב, ''Universitat Tel Aviv'') is a public research university in Tel Aviv, Israel. With over 30,000 students, it is the largest university in the country. Locate ...
in Israel
Israel (; he, יִשְׂרָאֵל, ; ar, إِسْرَائِيل, ), officially the State of Israel ( he, מְדִינַת יִשְׂרָאֵל, label=none, translit=Medīnat Yīsrāʾēl; ), is a country in Western Asia. It is situated ...
was the head designer of Shippingport Atomic Power Station in Pennsylvania, the first American civilian reactor to breed thorium. He founded a consortium to develop thorium reactors, which included other laboratories: Raytheon Nuclear Inc. and Brookhaven National Laboratory in the United States, and the Kurchatov Institute
The Kurchatov Institute (russian: Национальный исследовательский центр «Курчатовский Институт», 'National Research Centre "Kurchatov Institute) is Russia's leading research and developmen ...
in Russia.
In the 21st century, thorium's potential for reducing nuclear proliferation and its waste
Waste (or wastes) are unwanted or unusable materials. Waste is any substance discarded after primary use, or is worthless, defective and of no use. A by-product, by contrast is a joint product of relatively minor economic value. A waste pr ...
characteristics led to renewed interest in the thorium fuel cycle. India has projected meeting as much as 30% of its electrical demands through thorium-based nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced b ...
by 2050. In February 2014, Bhabha Atomic Research Centre (BARC), in Mumbai
Mumbai (, ; also known as Bombay — the official name until 1995) is the capital city of the Indian state of Maharashtra and the ''de facto'' financial centre of India. According to the United Nations, as of 2018, Mumbai is the secon ...
, India, presented their latest design for a "next-generation nuclear reactor" that burns thorium as its fuel ore, calling it the Advanced Heavy Water Reactor (AHWR). In 2009, the chairman of the Indian Atomic Energy Commission said that India has a "long-term objective goal of becoming energy-independent based on its vast thorium resources."
Nuclear weapons
When gram quantities ofplutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
were first produced in the Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
, it was discovered that a minor isotope ( 240Pu) underwent significant spontaneous fission, which brought into question the viability of a plutonium-fueled gun-type nuclear weapon. While the Los Alamos team began work on the implosion-type weapon to circumvent this issue, the Chicago team discussed reactor design solutions. Eugene Wigner proposed to use the 240Pu-contaminated plutonium to drive the conversion of thorium into 233U in a special converter reactor. It was hypothesized that the 233U would then be usable in a gun-type weapon, though concerns about contamination from 232U were voiced. Progress on the implosion weapon was sufficient, and this converter was not developed further, but the design had enormous influence on the development of nuclear energy. It was the first detailed description of a highly enriched water-cooled, water-moderated reactor similar to future naval and commercial power reactors.
During the Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because t ...
the United States explored the possibility of using 232Th as a source of 233U to be used in a nuclear bomb; they fired a test bomb in 1955. They concluded that a 233U-fired bomb would be a very potent weapon, but it bore few sustainable "technical advantages" over the contemporary uranium–plutonium bombs, especially since 233U is difficult to produce in isotopically pure form.
Thorium metal was used in the radiation case of at least one nuclear weapon design deployed by the United States (the W71
The W-71 nuclear warhead was a US thermonuclear warhead developed at Lawrence Livermore National Laboratory in California and deployed on the LIM-49A Spartan missile, a component of the Safeguard Program, an anti-ballistic missile (ABM) defense ...
).
Production
The common production route of thorium constitutes concentration of thorium minerals; extraction of thorium from the concentrate; purification of thorium; and (optionally) conversion to compounds, such as thorium dioxide.
 When added to
When added to
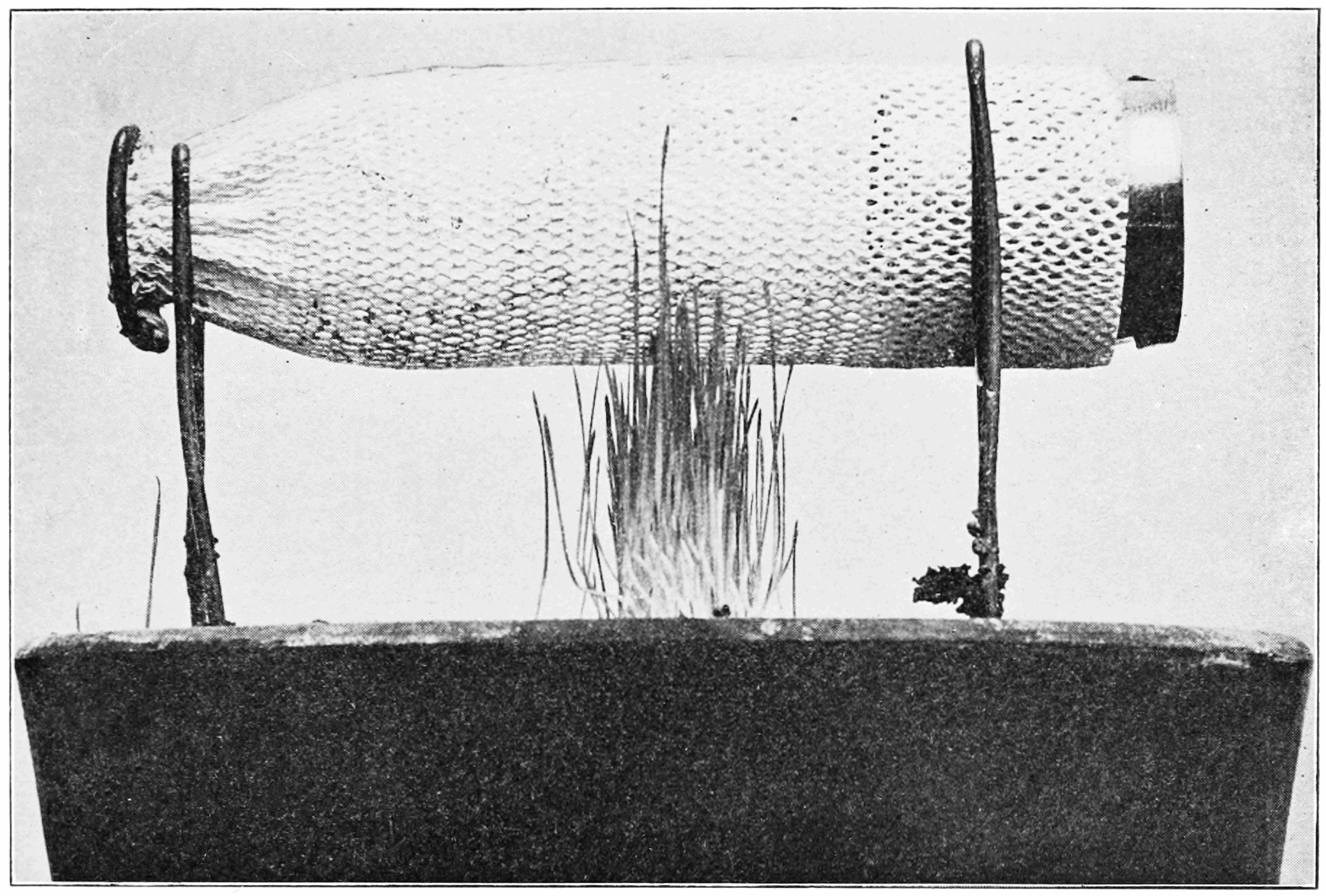
Thorium fuel cycle – Potential benefits and challenges
Concentration
There are two categories of thorium minerals for thorium extraction: primary and secondary. Primary deposits occur in acidic granitic magmas and pegmatites. They are concentrated, but of small size. Secondary deposits occur at the mouths of rivers in granitic mountain regions. In these deposits, thorium is enriched along with other heavy minerals. Initial concentration varies with the type of deposit. For the primary deposits, the source pegmatites, which are usually obtained by mining, are divided into small parts and then undergo flotation. Alkaline earth metal carbonates may be removed after reaction withhydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chlorid ...
; then follow thickening, filtration, and calcination. The result is a concentrate with rare-earth content of up to 90%. Secondary materials (such as coastal sands) undergo gravity separation. Magnetic separation follows, with a series of magnets of increasing strength. Monazite obtained by this method can be as pure as 98%.
Industrial production in the 20th century relied on treatment with hot, concentrated sulfuric acid in cast iron vessels, followed by selective precipitation by dilution with water, as on the subsequent steps. This method relied on the specifics of the technique and the concentrate grain size; many alternatives have been proposed, but only one has proven effective economically: alkaline digestion with hot sodium hydroxide solution. This is more expensive than the original method but yields a higher purity of thorium; in particular, it removes phosphates from the concentrate.
Acid digestion
Acid digestion is a two-stage process, involving the use of up to 93%sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
at 210–230 °C. First, sulfuric acid in excess of 60% of the sand mass is added, thickening the reaction mixture as products are formed. Then, fuming sulfuric acid is added and the mixture is kept at the same temperature for another five hours to reduce the volume of solution remaining after dilution. The concentration of the sulfuric acid is selected based on reaction rate and viscosity, which both increase with concentration, albeit with viscosity retarding the reaction. Increasing the temperature also speeds up the reaction, but temperatures of 300 °C and above must be avoided, because they cause insoluble thorium pyrophosphate to form. Since dissolution is very exothermic, the monazite sand cannot be added to the acid too quickly. Conversely, at temperatures below 200 °C the reaction does not go fast enough for the process to be practical. To ensure that no precipitates form to block the reactive monazite surface, the mass of acid used must be twice that of the sand, instead of the 60% that would be expected from stoichiometry. The mixture is then cooled to 70 °C and diluted with ten times its volume of cold water, so that any remaining monazite sinks to the bottom while the rare earths and thorium remain in solution. Thorium may then be separated by precipitating it as the phosphate at pH 1.3, since the rare earths do not precipitate until pH 2.
Alkaline digestion
Alkaline digestion is carried out in 30–45% sodium hydroxide solution at about 140 °C for about three hours. Too high a temperature leads to the formation of poorly soluble thorium oxide and an excess of uranium in the filtrate, and too low a concentration of alkali leads to a very slow reaction. These reaction conditions are rather mild and require monazite sand with a particle size under 45 μm. Following filtration, the filter cake includes thorium and the rare earths as their hydroxides, uranium as sodium diuranate, and phosphate as trisodium phosphate. This crystallises trisodium phosphate decahydrate when cooled below 60 °C; uranium impurities in this product increase with the amount ofsilicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
in the reaction mixture, necessitating recrystallisation before commercial use. The hydroxides are dissolved at 80 °C in 37% hydrochloric acid. Filtration of the remaining precipitates followed by addition of 47% sodium hydroxide results in the precipitation of thorium and uranium at about pH 5.8. Complete drying of the precipitate must be avoided, as air may oxidise cerium from the +3 to the +4 oxidation state, and the cerium(IV) formed can liberate free chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
from the hydrochloric acid. The rare earths again precipitate out at higher pH. The precipitates are neutralised by the original sodium hydroxide solution, although most of the phosphate must first be removed to avoid precipitating rare-earth phosphates. Solvent extraction may also be used to separate out the thorium and uranium, by dissolving the resultant filter cake in nitric acid. The presence of titanium hydroxide is deleterious as it binds thorium and prevents it from dissolving fully.
Purification
High thorium concentrations are needed in nuclear applications. In particular, concentrations of atoms with high neutron capture cross-sections must be very low (for example, gadolinium concentrations must be lower than one part per million by weight). Previously, repeated dissolution and recrystallisation was used to achieve high purity. Today, liquid solvent extraction procedures involving selective complexation of are used. For example, following alkaline digestion and the removal of phosphate, the resulting nitrato complexes of thorium, uranium, and the rare earths can be separated by extraction with tributyl phosphate inkerosene
Kerosene, paraffin, or lamp oil is a combustible hydrocarbon liquid which is derived from petroleum. It is widely used as a fuel in aviation as well as households. Its name derives from el, κηρός (''keros'') meaning " wax", and was re ...
.
Modern applications
Non-radioactivity-related uses of thorium have been in decline since the 1950s due to environmental concerns largely stemming from the radioactivity of thorium and its decay products. Most thorium applications use its dioxide (sometimes called "thoria" in the industry), rather than the metal. This compound has a melting point of 3300 °C (6000 °F), the highest of all known oxides; only a few substances have higher melting points. This helps the compound remain solid in a flame, and it considerably increases the brightness of the flame; this is the main reason thorium is used in gas lamp mantles. All substances emit energy (glow) at high temperatures, but the light emitted by thorium is nearly all in thevisible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelengths is called '' visible light'' or simply light. A typical human eye will respond to ...
, hence the brightness of thorium mantles.
Energy, some of it in the form of visible light, is emitted when thorium is exposed to a source of energy itself, such as a cathode ray, heat, or ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiatio ...
. This effect is shared by cerium dioxide, which converts ultraviolet light into visible light more efficiently, but thorium dioxide gives a higher flame temperature, emitting less infrared light. Thorium in mantles, though still common, has been progressively replaced with yttrium since the late 1990s. According to the 2005 review by the United Kingdom's National Radiological Protection Board, "although horiated gas mantleswere widely available a few years ago, they are not any more." Thorium is also used to make cheap permanent negative ion generator
An air ioniser (or negative ion generator or Chizhevsky's chandelier) is a device that uses high voltage to ionization, ionise (electrically charge) Atmosphere of Earth, air molecules. Negative ions, or anions, are particles with one or more ext ...
s, such as in pseudoscientific health bracelets.
During the production of incandescent
Incandescence is the emission of electromagnetic radiation (including visible light) from a hot body as a result of its high temperature. The term derives from the Latin verb ''incandescere,'' to glow white. A common use of incandescence i ...
filaments, recrystallisation of tungsten is significantly lowered by adding small amounts of thorium dioxide to the tungsten sintering
Clinker nodules produced by sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction.
Sintering happens as part of a manufacturing ...
powder before drawing the filaments. A small addition of thorium to tungsten thermocathodes considerably reduces the work function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately ...
of electrons; as a result, electrons are emitted at considerably lower temperatures. Thorium forms a one-atom-thick layer on the surface of tungsten. The work function from a thorium surface is lowered possibly because of the electric field on the interface between thorium and tungsten formed due to thorium's greater electropositivity. Since the 1920s, thoriated tungsten wires have been used in electronic tubes and in the cathodes and anticathodes of X-ray tubes and rectifiers. Thanks to the reactivity of thorium with atmospheric oxygen and nitrogen, thorium also acts as a getter for impurities in the evacuated tubes. The introduction of transistors in the 1950s significantly diminished this use, but not entirely. Thorium dioxide is used in gas tungsten arc welding (GTAW) to increase the high-temperature strength of tungsten electrodes and improve arc stability. Thorium oxide is being replaced in this use with other oxides, such as those of zirconium, cerium, and lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between l ...
.
Thorium dioxide is found in heat-resistant ceramics, such as high-temperature laboratory crucibles, either as the primary ingredient or as an addition to zirconium dioxide. An alloy of 90% platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
and 10% thorium is an effective catalyst for oxidising ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
to nitrogen oxides, but this has been replaced by an alloy of 95% platinum and 5% rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
because of its better mechanical properties and greater durability.
 When added to
When added to glass
Glass is a non- crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenchin ...
, thorium dioxide helps increase its refractive index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.
The refractive index determines how much the path of light is bent, ...
and decrease dispersion
Dispersion may refer to:
Economics and finance
*Dispersion (finance), a measure for the statistical distribution of portfolio returns
*Price dispersion, a variation in prices across sellers of the same item
*Wage dispersion, the amount of variatio ...
. Such glass finds application in high-quality lenses for cameras and scientific instruments. The radiation from these lenses can darken them and turn them yellow over a period of years and it degrades film, but the health risks are minimal. Yellowed lenses may be restored to their original colourless state by lengthy exposure to intense ultraviolet radiation. Thorium dioxide has since been replaced in this application by rare-earth oxides, such as lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between l ...
, as they provide similar effects and are not radioactive.
Thorium tetrafluoride is used as an anti-reflection material in multilayered optical coatings. It is transparent to electromagnetic waves having wavelengths in the range of 0.350–12 µm, a range that includes near ultraviolet, visible and mid infrared light. Its radiation is primarily due to alpha particles, which can be easily stopped by a thin cover layer of another material. Replacements for thorium tetrafluoride are being developed as of the 2010s, which include Lanthanum trifluoride.
Mag-Thor alloys (also called thoriated magnesium) found use in some aerospace applications, though such uses have been phased out due to concerns over radioactivity.
Potential use for nuclear energy
The main nuclear power source in a reactor is the neutron-induced fission of a nuclide; the synthetic fissile nuclei 233U and 239Pu can be bred from neutron capture by the naturally occurring quantity nuclides 232Th and 238U. 235U occurs naturally and is also fissile. In the thorium fuel cycle, the fertile isotope 232Th is bombarded by slow neutrons, undergoing neutron capture to become 233Th, which undergoes two consecutive beta decays to become first 233Pa and then the fissile 233U: : + 3 n → + + 2 n + n 233U is fissile and can be used as a nuclear fuel in the same way as 235U or 239Pu. When 233U undergoes nuclear fission, the neutrons emitted can strike further 232Th nuclei, continuing the cycle. This parallels the uranium fuel cycle in fast breeder reactors where 238U undergoes neutron capture to become 239U, beta decaying to first 239Np and then fissile 239Pu.Advantages
Thorium is more abundant than uranium, and can satisfy world energy demands for longer. It is particularly suitable for being used as fertile material in molten salt reactors. 232Th absorbs neutrons more readily than 238U, and 233U has a higher probability of fission upon neutron capture (92.0%) than 235U (85.5%) or 239Pu (73.5%). It also releases more neutrons upon fission on average. A single neutron capture by 238U produces transuranic waste along with the fissile 239Pu, but 232Th only produces this waste after five captures, forming 237Np. This number of captures does not happen for 98–99% of the 232Th nuclei because the intermediate products 233U or 235U undergo fission, and fewer long-lived transuranics are produced. Because of this, thorium is a potentially attractive alternative to uranium in mixed oxide fuels to minimise the generation of transuranics and maximise the destruction ofplutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
.
Thorium fuels result in a safer and better-performing reactor core because thorium dioxide has a higher melting point, higher thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
, and a lower coefficient of thermal expansion. It is more stable chemically than the now-common fuel uranium dioxide, because the latter oxidises to triuranium octoxide (), becoming substantially less dense.
Disadvantages
The used fuel is difficult and dangerous to reprocess because many of the daughters of 232Th and 233U are strong gamma emitters. All 233U production methods result in impurities of 232U, either from parasitic knock-out (n,2n) reactions on 232Th, 233Pa, or 233U that result in the loss of a neutron, or from double neutron capture of 230Th, an impurity in natural 232Th: : + n → + + n → + 232U by itself is not particularly harmful, but quickly decays to produce the strong gamma emitter 208Tl. (232Th follows the same decay chain, but its much longer half-life means that the quantities of 208Tl produced are negligible.) These impurities of 232U make 233U easy to detect and dangerous to work on, and the impracticality of their separation limits the possibilities of nuclear proliferation using 233U as the fissile material. 233Pa has a relatively long half-life of 27 days and a high cross section for neutron capture. Thus it is a neutron poison: instead of rapidly decaying to the useful 233U, a significant amount of 233Pa converts to 234U and consumes neutrons, degrading the reactor efficiency. To avoid this, 233Pa is extracted from the active zone of thorium molten salt reactors during their operation, so that it does not have a chance to capture a neutron and will only decay to 233U. The irradiation of 232Th with neutrons, followed by its processing, need to be mastered before these advantages can be realised, and this requires more advanced technology than the uranium and plutonium fuel cycle; research continues in this area. Others cite the low commercial viability of the thorium fuel cycle: the international Nuclear Energy Agency predicts that the thorium cycle will never be commercially viable while uranium is available in abundance—a situation which may persist "in the coming decades". The isotopes produced in the thorium fuel cycle are mostly not transuranic, but some of them are still very dangerous, such as 231Pa, which has a half-life of 32,760 years and is a major contributor to the long-term radiotoxicity of spent nuclear fuel.Hazards

Radiological
Natural thorium decays very slowly compared to many other radioactive materials, and the emitted alpha radiation cannot penetrate human skin. As a result, handling small amounts of thorium, such as those in gas mantles, is considered safe, although the use of such items may pose some risks. Exposure to an aerosol of thorium, such as contaminated dust, can lead to increased risk ofcancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
s of the lung
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of ...
, pancreas
The pancreas is an organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine gland, i.e. it has both an ...
, and blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the cir ...
, as lungs and other internal organs can be penetrated by alpha radiation. Internal exposure to thorium leads to increased risk of liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it i ...
diseases.
The decay products of 232Th include more dangerous radionuclides such as radium and radon. Although relatively little of those products are created as the result of the slow decay of thorium, a proper assessment of the radiological toxicity of 232Th must include the contribution of its daughters, some of which are dangerous gamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter r ...
emitters, and which are built up quickly following the initial decay of 232Th due to the absence of long-lived nuclides along the decay chain. As the dangerous daughters of thorium have much lower melting points than thorium dioxide, they are volatilised every time the mantle is heated for use. In the first hour of use large fractions of the thorium daughters 224Ra, 228Ra, 212Pb, and 212Bi are released. Most of the radiation dose by a normal user arises from inhaling the radium, resulting in a radiation dose of up to 0.2 millisieverts
The sievert (symbol: SvNot be confused with the sverdrup or the svedberg, two non-SI units that sometimes use the same symbol.) is a unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizing rad ...
per use, about a third of the dose sustained during a mammogram.
Some nuclear safety agencies make recommendations about the use of thorium mantles and have raised safety concerns regarding their manufacture and disposal; the radiation dose from one mantle is not a serious problem, but that from many mantles gathered together in factories or landfills is.
Biological
Thorium is odourless and tasteless. The chemical toxicity of thorium is low because thorium and its most common compounds (mostly the dioxide) are poorly soluble in water, precipitating out before entering the body as the hydroxide. Some thorium compounds are chemically moderatelytoxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a sub ...
, especially in the presence of strong complex-forming ions such as citrate that carry the thorium into the body in soluble form. If a thorium-containing object has been chewed or sucked, it loses 0.4% of thorium and 90% of its dangerous daughters to the body. Three-quarters of the thorium that has penetrated the body accumulates in the skeleton
A skeleton is the structural frame that supports the body of an animal. There are several types of skeletons, including the exoskeleton, which is the stable outer shell of an organism, the endoskeleton, which forms the support structure inside ...
. Absorption through the skin is possible, but is not a likely means of exposure. Thorium's low solubility in water also means that excretion of thorium by the kidneys and faeces is rather slow.
Tests on the thorium uptake of workers involved in monazite processing showed thorium levels above recommended limits in their bodies, but no adverse effects on health were found at those moderately low concentrations. No chemical toxicity has yet been observed in the tracheobronchial tract
The respiratory tract is the subdivision of the respiratory system involved with the process of Respiration (physiology), respiration in mammals. The respiratory tract is lined with respiratory epithelium as respiratory mucosa.
Air is breathed ...
and the lungs from exposure to thorium. People who work with thorium compounds are at a risk of dermatitis
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved c ...
. It can take as much as thirty years after the ingestion of thorium for symptoms to manifest themselves. Thorium has no known biological role.
Chemical
Powdered thorium metal is pyrophoric: it ignites spontaneously in air. In 1964, theUnited States Department of the Interior
The United States Department of the Interior (DOI) is one of the executive departments of the U.S. federal government headquartered at the Main Interior Building, located at 1849 C Street NW in Washington, D.C. It is responsible for the ma ...
listed thorium as "severe" on a table entitled "Ignition and explosibility of metal powders". Its ignition temperature was given as 270 °C (520 °F) for dust clouds and 280 °C (535 °F) for layers. Its minimum explosive concentration was listed as 0.075 oz/cu ft (0.075 kg/m3); the minimum igniting energy for (non-submicron) dust was listed as 5 mJ.
In 1956, the Sylvania Electric Products explosion
On the morning of July 2, 1956, three explosions involving scrap thorium occurred at the Sylvania Electric Products' Metallurgical Laboratory in Bayside, (now Bay Terrace) Queens, New York. Nine people were injured, some severely. One 28 year ...
occurred during reprocessing and burning of thorium sludge in New York City
New York, often called New York City or NYC, is the List of United States cities by population, most populous city in the United States. With a 2020 population of 8,804,190 distributed over , New York City is also the L ...
, United States. Nine people were injured; one died of complications caused by third-degree burns.
Exposure routes
Thorium exists in very small quantities everywhere on Earth although larger amounts exist in certain parts: the average human contains about 40 micrograms of thorium and typically consumes three micrograms per day. Most thorium exposure occurs through dust inhalation; some thorium comes with food and water, but because of its low solubility, this exposure is negligible. Exposure is raised for people who live near thorium deposits or radioactive waste disposal sites, those who live near or work in uranium, phosphate, or tin processing factories, and for those who work in gas mantle production. Thorium is especially common in theTamil Nadu
Tamil Nadu (; , TN) is a state in southern India. It is the tenth largest Indian state by area and the sixth largest by population. Its capital and largest city is Chennai. Tamil Nadu is the home of the Tamil people, whose Tamil language ...
coastal areas of India, where residents may be exposed to a naturally occurring radiation dose ten times higher than the worldwide average. It is also common in northern Brazil
Brazil ( pt, Brasil; ), officially the Federative Republic of Brazil (Portuguese: ), is the largest country in both South America and Latin America. At and with over 217 million people, Brazil is the world's fifth-largest country by area ...
ian coastal areas, from south Bahia
Bahia ( , , ; meaning "bay") is one of the 26 states of Brazil, located in the Northeast Region of the country. It is the fourth-largest Brazilian state by population (after São Paulo, Minas Gerais, and Rio de Janeiro) and the 5th-larges ...
to Guarapari, a city with radioactive monazite sand beaches, with radiation levels up to 50 times higher than world average background radiation.
Another possible source of exposure is thorium dust produced at weapons testing ranges, as thorium is used in the guidance systems of some missiles. This has been blamed for a high incidence of birth defects and cancer at Salto di Quirra
Salto di Quirra is a restricted weapons testing range and rocket launching site near Perdasdefogu on Sardinia.
It is the largest military range in Italy, composed of 12000 hectares of land owned by the Italian Ministry of Defence and one of the ...
on the Italian island of Sardinia
Sardinia ( ; it, Sardegna, label=Italian, Corsican and Tabarchino ; sc, Sardigna , sdc, Sardhigna; french: Sardaigne; sdn, Saldigna; ca, Sardenya, label= Algherese and Catalan) is the second-largest island in the Mediterranean Sea, aft ...
.
See also
*Thorium Energy Alliance
Thorium Energy Alliance (TEA) is a non-governmental, non-profit 501(c)3, educational organization based in the United States, which seeks to promote energy security of the world through the use of thorium as a fuel source. The potential for the ...
Explanatory notes
Citations
General bibliography
* * *Further reading
* * International Atomic Energy Agency (2005)Thorium fuel cycle – Potential benefits and challenges
External links
* * {{Authority control Chemical elements Chemical elements with face-centered cubic structure Actinides Carcinogens Nuclear fuels Nuclear materials