type II secretion system on:
[Wikipedia]
[Google]
[Amazon]
The type 2 secretion system (often referred to as the type II secretion system or by the initials T2SS) is a type of protein
 Overall the type II secretion system is a large multiprotein machinery, made up of a number of distinct protein subunits known as the general secretory proteins (GSPs). The
Overall the type II secretion system is a large multiprotein machinery, made up of a number of distinct protein subunits known as the general secretory proteins (GSPs). The
secretion
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mec ...
machinery found in various species of Gram-negative bacteria
Gram-negative bacteria are bacteria that, unlike gram-positive bacteria, do not retain the Crystal violet, crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is that their cell envelo ...
, including many human pathogens
In biology, a pathogen (, "suffering", "passion" and , "producer of"), in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ.
The term ...
such as ''Pseudomonas aeruginosa
''Pseudomonas aeruginosa'' is a common Bacterial capsule, encapsulated, Gram-negative bacteria, Gram-negative, Aerobic organism, aerobic–facultative anaerobe, facultatively anaerobic, Bacillus (shape), rod-shaped bacteria, bacterium that can c ...
'' and ''Vibrio cholerae
''Vibrio cholerae'' is a species of Gram-negative bacteria, Gram-negative, Facultative anaerobic organism, facultative anaerobe and Vibrio, comma-shaped bacteria. The bacteria naturally live in Brackish water, brackish or saltwater where they att ...
''. The type II secretion system is one of six protein secretory systems commonly found in Gram-negative bacteria, along with the type I, type III, and type IV secretion systems, as well as the chaperone/usher pathway, the autotransporter pathway/type V secretion system, and the type VI secretion system (some bacteria also utilize the type VII secretion system). Like these other systems, the type II secretion system enables the transport of cytoplasmic proteins across the lipid bilayers that make up the cell membranes of Gram-negative bacteria. Secretion of proteins and effector
Effector may refer to:
*Effector (biology), a molecule that binds to a protein and thereby alters the activity of that protein
* ''Effector'' (album), a music album by the Experimental Techno group Download
* ''EFFector'', a publication of the El ...
molecules out of the cell plays a critical role in signaling other cells and in the invasion and parasitism of host cells.
Overview
The type II secretion system is a membrane-boundprotein complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multidomain enzymes, in which multiple active site, catalytic domains are found in a single polypeptide chain.
...
found in Gram-negative
Gram-negative bacteria are bacteria that, unlike gram-positive bacteria, do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is that their cell envelope consists ...
bacteria that is used to secrete proteins found in the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
of the bacteria into the extracellular space
Extracellular space refers to the part of a multicellular organism outside the cells, usually taken to be outside the plasma membranes, and occupied by fluid. This is distinguished from intracellular space, which is inside the cells.
The composit ...
outside of the cell. The type II secretion system is just one of many secretory systems found in Gram-negative bacteria and is used to secrete a variety of different proteins, including bacterial toxins and degradative enzymes such as protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
s and lipase
In biochemistry, lipase ( ) refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; howe ...
s. These secreted proteins are generally associated with the breakdown of host tissues and therefore are often important in causing the symptoms associated with certain bacterial infections
Pathogenic bacteria are bacteria that can cause disease. This article focuses on the bacteria that are pathogenic to humans. Most species of bacteria are harmless and many are beneficial but others can cause infectious diseases. The number of t ...
. Each bacterial cell may contain a number of type II secretion complexes, which are found embedded in the inner and outer membranes of the cell.
Along with other secretory systems such as the chaperone/usher pathway and the type IV secretion system, type II secretion is a two-step process. The first step involves the Sec and Tat secretory pathways, which are responsible for transporting proteins across the inner membrane into the periplasm
The periplasm is a concentrated gel-like matrix in the space between the inner cytoplasmic membrane and the bacterial outer membrane called the ''periplasmic space'' in Gram-negative (more accurately "diderm") bacteria. Using cryo-electron micros ...
. For instance, the Sec pathway is used to transport structural components of the type II secretion system into the periplasm where they can then assemble, while both the Sec and Tat pathways are used to transport secretory proteins into the periplasm. Once these secretory proteins are located in the periplasm, the second step can then take place, whereby they are secreted across the outer membrane into the extracellular milieu.
Structure
genes
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
encoding these GSPs are usually found together in the genome
A genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding genes, other functional regions of the genome such as ...
in a single operon
In genetics, an operon is a functioning unit of DNA containing a cluster of genes under the control of a single promoter. The genes are transcribed together into an mRNA strand and either translated together in the cytoplasm, or undergo splic ...
and many of these genes overlap. Each gene is named with a letter corresponding to the GSP that it encodes (for example the gspD gene encodes GspD) and studies indicate that between 12 and 15 of these genes are essential to the function of the type II secretion system. The GSPs are common among a number of different bacterial species
A species () is often defined as the largest group of organisms in which any two individuals of the appropriate sexes or mating types can produce fertile offspring, typically by sexual reproduction. It is the basic unit of Taxonomy (biology), ...
and when they come together they form a complex that is structurally very similar to the type IV pili, an appendage that is also commonly found in gram negative bacteria. Overall the type II secretion system can be broken down into four main components. These are the outer membrane complex, the inner membrane complex, the secretion ATPase
ATPases (, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, ATP hydrolase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or ...
and the pseudopilus.
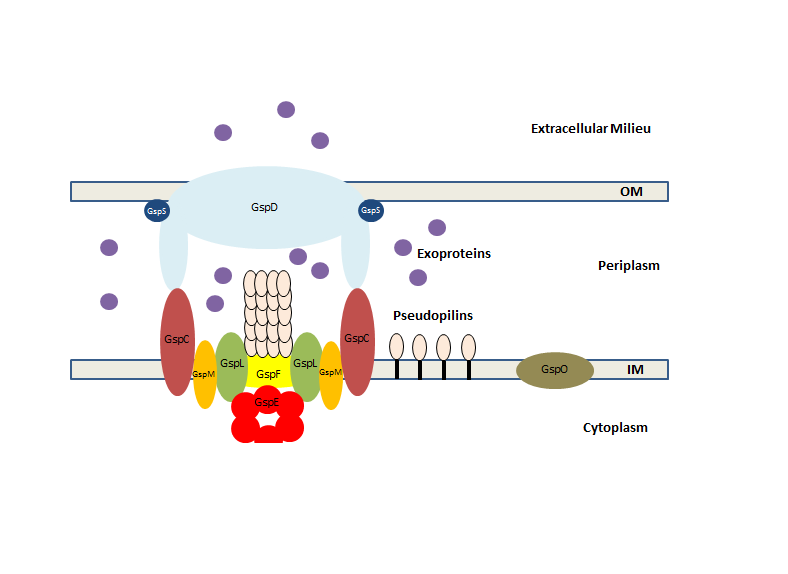
Outer Membrane Complex
The outer membrane complex is made up largely by the secretin GspD. Secretins are β-barrels that are found in membrane where they form channels that allow substances to move in or out of cells. In the type II secretion system GspD creates a pore in the outer membrane of the bacterial cell through which proteins can be secreted. As a result, GspD is essential for the correct function system because without it secretory proteins cannot exit the cell. GspD is transported into the periplasm via the Sec translocon and is then inserted into the outer membrane. This insertion is not spontaneous however and is often reliant upon the β-barrel assembly machinery which ensures β-barrel proteins are folded correctly before insertion into the membrane. GspD is often found associated with thelipoprotein
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid (also known as fat) molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, sur ...
GspS. GspS is also transported into the periplasm using the Sec translocation machinery, at which point it is inserted into the inner layer of the outer membrane where it remains closely associated with GspD. It is thought that GspS plays an important role in the stabilization of the secretin GspD and helps prevent it from breaking down in the presence of highly degradative periplasmic enzymes
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as pro ...
.
The translocation process of GspD to the outer membrane differs between GspD homologs. ''Escherichia coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly fo ...
'' secretins GspDα and GspDβ show distinct translocation mechanisms ''in vitro''. The GspDβ forms its multimer on the inner membrane and interacts with GspS (pilotin), which disassembles GspDβ into its monomers. The monomers are guided through the peptidoglycan layer by GspS and forms the GspDβ multimer on the outer membrane together with GspS, forming a GspDβ-GspS complex. GspDα however, can translocate to the outer membrane without the assistance of pilotin in the presence of D-methionine. In the case of GspDα, it forms its multimer on the inner membrane, where it exists in an unstable form shifting between two confirmations. Reduced cross-linking in the peptidoglycan, induced by D-methionine, causes the formation of pores in the peptidoglycan layer which allows GspDα to translocate to the outer membrane, where it exists in a more stable and connected confirmation.
Inner Membrane Complex
The inner membrane complex is made up of several different Gsp proteins which are embedded in the inner membrane. Like the outer membrane secretin GspD these proteins are transported into the periplasm via the Sec translocation pathway before being inserted into the inner membrane. Four different proteins make up the inner membrane complex; these are GspC, GspF, GspL and GspM. Each of these individual subunits plays a slightly different role. GspC for instance has been shown to interact with GspD. This interaction helps gate the type II secretion system and only when this gate is open are secretory proteins able to enter the system and be pumped out of the cell. Importantly, when associated together, GspC, GspL and GspM help protect each other from proteolytic enzymes that would otherwise degrade them. Unlike the other proteins that make up the inner membrane complex GspF is a multipass transmembrane protein and it may play a role in binding the secretion ATPase. GspL is however known to form tight interactions with the secretion ATPase and these are needed to hold it in close association with the rest of the inner membrane complex.Secretion ATPase
The secretion ATPase, GspE, is an ATPase which is found closely associated with the inner membrane complex on the cytoplasmic side of the inner membrane. GspE belongs to the type II/type IV secretion ATPase family. ATPases belonging to this family have a distinct hexameric structure. Each individual subunit of the hexamer has 3 main domains. These are 2 separateN-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
domains called N1D and N2D which are separated by a short linker region and a single C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When t ...
domain termed the CTD. The CTD in turn is made up of 3 subdomains, one of which is a nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
binding domain
In molecular biology, binding domain is a protein domain which binds to a specific atom or molecule, such as calcium or DNA. A protein domain is a part of a protein sequence and a tertiary structure that can change or evolve, function, and live ...
. It is this nucleotide binding domain, which is present in of each of the 6 subunits of the hexamer, that is responsible for binding ATP. The other 2 domains that make up the CTD, a four helical domain and a metal binding domain, then help catalyze
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of bound ATP. This ATP hydrolysis is used to power the assembly and disassembly of the pseudopillus which is what drives secretion via the type II secretion system. As a result, the system cannot function without GspE. The N-terminal domains N1D and N2D form the interactions with the inner membrane complex which help keep the secretion ATPase in close association with the rest of the type II secretion system. The N2D domain is not fully understood but observations show that it is the N1D which is responsible for forming the tight interactions seen with the inner membrane complex subunit GspL.
Pseudopilus
The pseudopilus is found in the periplasm but does not extend out through the secretin GspD into the extracellular milieu. Its name it derived from the fact that it is made up of a number of pilin like proteins or pseudopilins, known as GspG, GspH, GspI, GspJ and GspK. They are known as pseudopilins due to their similarity to the pilins (like PilA) that make up the type IV pili found in gram negative bacteria. Like their counterparts, the pseudopilins are initially produced in an immature form. These pre-pseudopilins consist of an N-terminal signal sequence that targets the proteins to the Sec translocon and a long C-terminal passenger domain which encodes the actual pseudopilin protein itself. Once the Sec machinery has transported the pre-pseudopilin across the inner membrane, but before the protein itself is released into the periplasm, the N-terminal signal sequence is cleaved at a conserved stretch of positively chargedamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
residues. This cleavage is catalysed by the signal peptidase
Signal peptidases are enzymes that convert secretory and some membrane proteins to their mature or pro forms by cleaving their signal peptides from their N-termini.
Signal peptidases were initially observed in endoplasmic reticulum (ER)-deri ...
GspO and the end result is the removal of the N-terminal signal sequence and the formation of a mature pseudopilin. GspO is inserted in the inner membrane and is often closely associated with the type II secretion system machinery. Mature pilins and pseudopilins have a lollipop-shaped structure, made up of a long hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
tail and a globular
A globular cluster is a spheroidal conglomeration of stars that is bound together by gravity, with a higher concentration of stars towards its center. It can contain anywhere from tens of thousands to many millions of member stars, all orbiting ...
hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
head domain. Once in the periplasm in their mature state, the pseudopilins will then often be inserted into the outer leaflet of the inner membrane via their hydrophobic tails.
The major pseudopilin present in the pseudopilus is GspG. The pseudopilus forms when the individual pseudopilin subunits polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
together. In this reaction the hydrophobic tails of different pseudopilins mesh together leaving their globular hydrophilic heads exposed. These long hydrophobic tails are able to aggregate together like this due to strong hydrophobic interactions and the end result is that the pseudopilus steadily grows. The assembly and disassembly of these pseudopilus subunits is powered by the secretion ATPase GspE. It is thought that this constant extension and retraction of the pseudopilus causes it to act like a piston
A piston is a component of reciprocating engines, reciprocating pumps, gas compressors, hydraulic cylinders and pneumatic cylinders, among other similar mechanisms. It is the moving component that is contained by a cylinder (engine), cylinder a ...
and push secretory proteins out through the outer membrane secretin. When the pseudopilus then retracts new secretory proteins can then enter the system and the process will repeat. This movement of the pseudopilus is similar to the movement displayed by type IV pili which is known to enable twitching motility
Twitch may refer to:
Biology
* Muscle contraction
** Convulsion, rapid and repeated muscle contraction and relaxation
** Fasciculation, a small, local, involuntary muscle contraction
** Myoclonic twitch, a jerk usually caused by sudden muscle co ...
.

Mechanism
Secretion of proteins via the type II secretion system occurs in a very specific way and is largely uniform among different species of bacteria. This mechanism can be broken down into several steps: #Exoproteins, or proteins that are to be secreted, are first transported across the inner membrane and into the periplasm via the Sec translocation machinery. These exoproteins will exist here in the periplasm secretion until the type II secretion system is activated. #Pre-pseudopilins are also transported from the cytoplasm into the periplasm via the Sec translocation machinery. Once in the periplasm they are cleaved by the pre-pilin peptidase GspO and converted into mature pseudopilins. The mature pseudopilins can then insert themselves into the inner membrane where they will exist until pseudopilus assembly occurs. #The secretion ATPase GspE will then bind and hydrolyze ATP and the energy produced is used to power the formation of the pseudopilus. GspE is located in the cytoplasm but remains associated with the inner membrane complex via interactions with both GspL and GspF. #When activated, the exoproteins previously transported into the periplasm are able to enter the secretion machinery. It is not fully understood how these exoproteins are selected for, but it is believed the interaction between GspC and GspD plays an important role. #The assembly of the pseudopilus then forces the exoproteins out through the secretin GspD and into the extracellular milieu. This secretin forms a hydrophilic channel in the outer membrane which allows the proteins to exit the cell. #Once outside of the cell the secreted exoproteins can then carry out their intended effects. Some of these for instance may be involved insignalling
A signal is both the process and the result of transmission of data over some media accomplished by embedding some variation. Signals are important in multiple subject fields including signal processing, information theory and biology.
In ...
and others may act as virulence factors that help promote infection.
It is believed that quorum sensing
In biology, quorum sensing or quorum signaling (QS) is the process of cell-to-cell communication that allows bacteria to detect and respond to cell population density by gene regulation, typically as a means of acclimating to environmental disadv ...
plays a key role in controlling the activation of the type II secretion system and the initiation of exoprotein release. Specifically quorum sensing helps regulate the transcription of the genes encoding these exoproteins and ensures that they are only produced when other like bacteria are nearby and environmental conditions are conducive to survival and infection.
References
{{Reflist Bacteriology Secretion