Troc Group Protection on:
[Wikipedia]
[Google]
[Amazon]
Trichloroethyl chloroformate is used in
 2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a
2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a
 *
*  *
* 
organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
for the introduction of the trichloroethyl chloroformate (Troc) protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
for amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s, thiols
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
and alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
. It readily cleaves vs other carbamates and can be used in an overall protecting group strategy.
The troc group is traditionally removed ''via'' Zn insertion in the presence of acetic acid, resulting in elimination and decarboxylation.
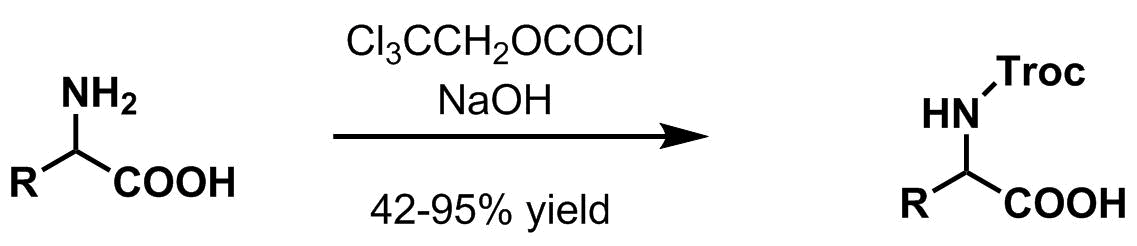
Amine protection – 2,2,2-Trichloroethoxycarbonyl (Troc)
: 2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a
2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
for amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
.
Most common amine protection methods
* 2,2,2-Trichloroethyl chloroformate,pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
or aqueous sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
at ambient temperature
: *
* Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
: *
* Deprotection using zinc metal
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In man ...
:
References
{{DEFAULTSORT:Trichlorethoxycarbonyl chloride, 2,2,2- Chloroformates Protecting groups Reagents for organic chemistry