Trimerisation on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, a trimer (; ) is a
 In 1866,
In 1866,
 The bromide has an extended shelflife when refrigerated. Like the chloride, it undergoes ab exothermic trimerisation to form
The bromide has an extended shelflife when refrigerated. Like the chloride, it undergoes ab exothermic trimerisation to form 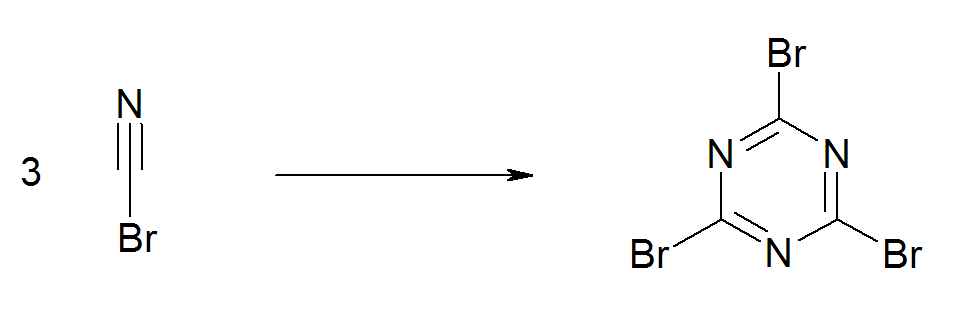 An industrial route to
An industrial route to 3 H2N-CO-NH2 -> (O)NH + 3 NH3
The endothermic synthesis of (NH2)2CO -> HOCN + NH3
Then in the second step, cyanic acid polymerizes to form cyanuric acid, which condenses with the liberated ammonia from the first step to release melamine and water.
: 3 HOCN -> (O)NH
: (O)NH + 3 NH3 -> C3H6N6 + 3 H2O
This water then reacts with cyanic acid present, which helps drive the trimerization reaction, generating carbon dioxide and ammonia.
: 3 HOCN + 3 H2O -> 3 CO2 + 3NH3
In total, the second step is 6 HCNO + 3 NH3 -> C3H6N6 + 3 CO2 + 3NH3
but the overall process is
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relat ...
derived from three identical precursors often in competition with polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
.
Examples
Alkyne trimerisation
Marcellin Berthelot
Pierre Eugène Marcellin Berthelot (; 25 October 1827 – 18 March 1907) was a French chemist and Republican politician noted for the ThomsenBerthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substanc ...
reported the first example of cyclotrimerization, the conversion of acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pur ...
to benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
. This process was commercialized:
:
Nitrile trimerization
Symmetrical 1,3,5-triazines are prepared by trimerization of certainnitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including me ...
s such as cyanogen chloride
Cyanogen chloride is a highly toxic chemical compound with the formula CNCl. This linear, triatomic pseudohalogen is an easily condensed colorless gas. More commonly encountered in the laboratory is the related compound cyanogen bromide, a room-t ...
or cyanimide.
Cyanogen chloride
Cyanogen chloride is a highly toxic chemical compound with the formula CNCl. This linear, triatomic pseudohalogen is an easily condensed colorless gas. More commonly encountered in the laboratory is the related compound cyanogen bromide, a room-t ...
and cyanogen bromide
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. ...
each trimerize at elevated temperatures over a carbon catalyst. The chloride gives cyanuric chloride
Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controvers ...
:
:cyanuric bromide
Cyanuric bromide is a heterocyclic compound with formula C3N3Br3. It contains a six-membered ring of alternating nitrogen and carbon atoms, with a bromine atom attached to each carbon. It is formed by the spontaneous trimerisation of cyanogen brom ...
. This reaction is catalyzed by traces of bromine, metal salts, acids and bases. For this reason, experimentalists avoid brownish samples.
:cyanuric acid
Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula (CNOH)3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleac ...
entails the thermal decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
of urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
, with release of ammonia. The conversion commences at approximately 175 °C:Klaus Huthmacher, Dieter Most "Cyanuric Acid and Cyanuric Chloride" Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. doi 10.1002/14356007.a08 191
: melamine
Melamine is an organic compound with the formula C3H6N6. This white solid is a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 67% nitrogen by mass, and its derivatives have fire retardant properties due t ...
can be understood in two steps.
:
First, urea decomposes into cyanic acid and ammonia in an endothermic reaction:
: exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity ...
:
: endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. ...
.
Diene trimerisation
The 1,5,9-trans-trans-cis isomer of cyclododecatriene, which has some industrial importance is obtained by cyclotrimerization ofbutadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vin ...
with titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula . It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. is a volatile liquid. Upon contact with humid air, it forms thick clouds ...
and an organoaluminium co-catalyst:''Industrial Organic Chemistry'', Klaus Weissermel, Hans-Jurgen Arpe John Wiley & Sons; 3rd 1997
:
Breaking carbon-hetero double bonds forms symmetrical saturated 1,3,5-heterocycles
Cyclotrimerization offormaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
affords 1,3,5-Trioxane:
:
1,3,5-Trithiane is the cyclic trimer of the otherwise unstable species thioformaldehyde. This heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
consists of a six-membered ring with alternating methylene bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of ...
s and thioether groups. It is prepared by treatment of formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
with hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
.
Three molecules of acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
condense to form paraldehyde
Paraldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane, with a methyl group substituted for a hydrogen atom at each carbon. The corresponding tetramer is metaldehyde. A colourless liquid, it ...
, a cyclic trimer containing C-O single bonds.
Catalyzing and dehydrating by sulfuric acid, trimerization of acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscible wi ...
via aldol condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration ...
affords mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
Trisiloxanes
Dimethylsilanediol dehydrates to a trimer of as well aspolydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, as it ...
. The reaction illustrates the competition between trimerization and polymerization. The polymer and trimer are formally derived from the hypothetical sila-ketone , although this species is not an intermediate.
Coordination chemistry
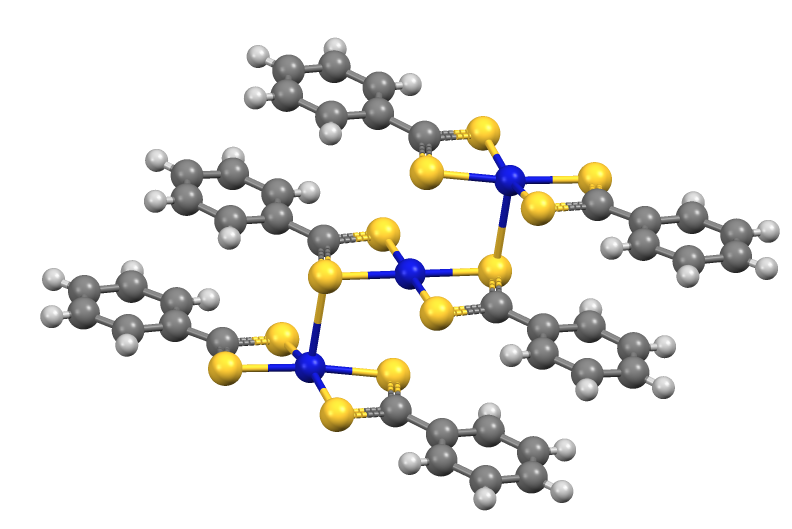
The dithiobenzoate complexes crystallize as trimers (M = Ni, Pd).
See also
*Oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relat ...
* Protein trimer
References
{{DEFAULTSORT:Trimer (Chemistry) Trimers (chemistry)