Triacetic Acid Lactone on:
[Wikipedia]
[Google]
[Amazon]
Triacetic acid lactone (TAL; 4-hydroxy-6-methyl-2-pyrone) is an organic compound derived enzymatically from glucose. It is a light yellow solid that is soluble in organic solvents.
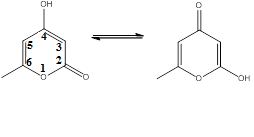 The tautomer on the left, featuring a 4-hydroxy group, the C4 carbon, is dominant. Triacetic acid lactone is classified as a 2-pyrone compound owing to the ketone group on the C2 carbon in its dominant form.
The tautomer on the left, featuring a 4-hydroxy group, the C4 carbon, is dominant. Triacetic acid lactone is classified as a 2-pyrone compound owing to the ketone group on the C2 carbon in its dominant form.

Structure
Triacetic acid lactone consists of two main tautomers. The tautomer on the left, featuring a 4-hydroxy group, the C4 carbon, is dominant. Triacetic acid lactone is classified as a 2-pyrone compound owing to the ketone group on the C2 carbon in its dominant form.
The tautomer on the left, featuring a 4-hydroxy group, the C4 carbon, is dominant. Triacetic acid lactone is classified as a 2-pyrone compound owing to the ketone group on the C2 carbon in its dominant form.
Synthesis
Triacetic acid lactone is synthesized either fromdehydroacetic acid
Dehydroacetic acid is an organic compound which has several industrial applications. The compound is classified as a pyrone derivative. It presents as an odorless, colorless to white crystalline powder, almost insoluble in water and moderately so ...
, another 2-pyrone derivative, or from glucose by enzymatic catalysis. In its original synthesis, triacetic acid lactone was obtained by treatment of dehydroacetic acid with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
at 135 °C. Dehydroacetic acid undergoes ring-opening and hydration to form "tetracetic acid". Upon cooling, triacetic acid reverts to a lactone ring similar to the dehydroacetic acid structure, and the triacetic acid lactone is recovered by crystallization in cold water.

Biosynthesis
The microbial synthesis of triacetic acid lactone requires the enzyme 2-pyrone synthase (2-PS). This enzyme has been examined in two hosts Escherichia coli and Saccharomyces cerevisiae. The Saccharomyces cerevisiae host being used during the synthesis produces a higher yield (70%) compared with the Escherichia coli host, which produces a yield of 40% of triacetic acid lactone. This enzyme catalyzes the synthesis of triacetic acid lactone fromacetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
via two subsequent condensations with malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Functions
It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Fatty acid biosynthesis
Malonyl-CoA provides 2-carbon units to fatty acids and commi ...
. This produces an intermediate of 3,5-diketohexanoate thioester, which undergoes ring closure to produce triacetic acid lactone.
Reactivity
The lactone is a versatile intermediate inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. It has also been described as a platform chemical, meaning that it could be the precursor to other fine chemicals. The lactone undergoes decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
to acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are tre ...
. It is also a precursor to sorbic acid, dienoic acid, and hexenoic acid. Dienoic acid is used to inhibit the growth of various molds and hexenoic acid is used as a flavoring agent. Acetylacetone is used for metal extraction and plating and as a food additive.{{cite journal , doi = 10.1039/C2GC35343A , title = Triacetic acid lactone as a potential biorenewable platform chemical , year = 2012 , last1 = Chia , first1 = Mei , last2 = Schwartz , first2 = Thomas J. , last3 = Shanks , first3 = Brent H. , last4 = Dumesic , first4 = James A. , journal = Green Chemistry , volume = 14 , issue = 7 , pages = 1850
References