Transition Metal Imidazole Complex on:
[Wikipedia]
[Google]
[Amazon]
 A transition metal imidazole complex is a
A transition metal imidazole complex is a
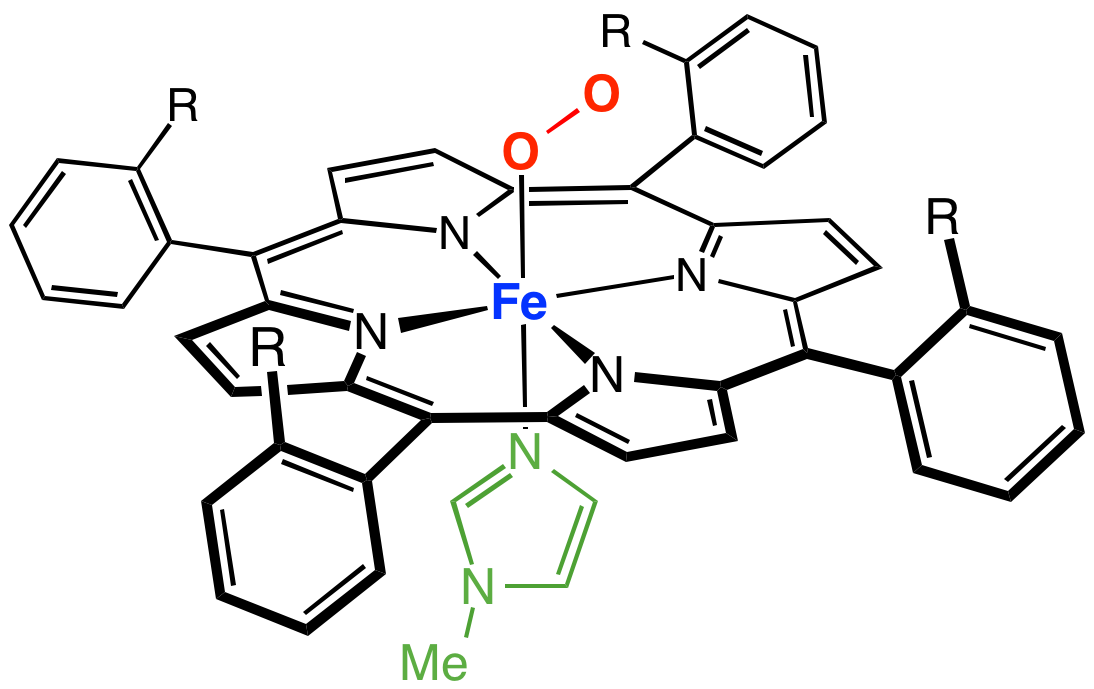 Only the
Only the
 The pKa of imidazole (to give imidazolate) is 14, thus it is easy to deprotonate. Many metal complexes feature imidazolate as a
The pKa of imidazole (to give imidazolate) is 14, thus it is easy to deprotonate. Many metal complexes feature imidazolate as a
 A transition metal imidazole complex is a
A transition metal imidazole complex is a coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. ...
that has one or more imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
s. Complexes of imidazole itself are of little practical importance. In contrast, imidazole derivatives, especially histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
, are pervasive ligands in biology where they bind metal cofactors.
Bonding and structure
: Only the
Only the imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
nitrogen (HC=N-CH) of imidazole is basic, and it is this nitrogen that binds to metal ions. The pyrrole-like nitrogen ((HC-NH-CH) projects away from the metal. The pKa of protonated imidazolium
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
cation is about 6.95, which indicates that the basicity of imidazole is intermediate between pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ...
(pKa of pyridinium = 5.23) and ammonia (pKa = 9,24 of ammonium). The donor properties of imidazole are also indicated by the redox properties of its complexes.
Imidazole is a pure sigma-donor ligand. There is no evidence for pi backbonding
In chemistry, π backbonding, also called π backdonation, is when electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a ''π-acceptor ligand''. It is especially common in the organometallic chemi ...
in metal-imidazole complexes, a property that can be attributed to the presence of the pi-donor pyrrole-like NH center. For this reason, imidazole can be classified as hard
Hard may refer to:
* Hardness, resistance of physical materials to deformation or fracture
* Hard water, water with high mineral content
Arts and entertainment
* ''Hard'' (TV series), a French TV series
* Hard (band), a Hungarian hard rock supe ...
ligand. Nonetheless, complexes between low-valent metals and imidazole are well known, e.g., e(imidazole)3(CO)3sup>+.
Imidazole is a compact, flat ligand. Six imidazole ligands fit comfortably around octahedral metal centers, e.g., e(imidazole)6sup>2+. The M-N(imidazole) bond is freely rotating.
Homoleptic octahedral complexes have been characterized by X-ray crystallography for the following dications: Fe2+, Co2+, Ni2+, Zn2+, Cd2+. Hexakis complexes of both Ru2+ and Ru3+ are also known. Cu2+, Pd2+, and Pt2+ form homoleptic square planar complexes. Zn2+, although crystallized as the hexakis complex, more typically forms a tetrahedral complex.
Complexes of substituted imidazoles
Structure of vitamin b12, illustrating the dimethylbenzimidazole ligand.N-methylimidazole
1-Methylimidazole or ''N''-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundament ...
is slightly more basic than imidazole but is otherwise similar, if more lipophilic. Many salts of (imidazole-1-R)6sup>2+ are known (R = alkyl, vinyl, etc.). 2-Methylimidazoles are somewhat bulky ligands owing to the steric clash between the 2-methyl group and other ligands in octahedral complexes.
A modified benzimidazole
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a colorless solid.
Preparation
Benzimidazole is produced by condensation of o ...
ligand is found in all versions of vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. It is one of eight B vitamins. It is required by animals, which use it as a cofactor in DNA synthesis, in both fatty acid and amino acid metabolism. ...
.
Histidine
Histidine complexes comprise an important subset oftransition metal amino acid complexes Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the trans ...
. In common with other 3-substituted imidazoles, histidine can coordinate to metals via either of two nonequivalent tautomers
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
. The free amino acid can coordinate through the imidazole and either or both of the carboxylate and amine.
The imidazole side chain of histidine residues in protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s are common binding sites for metal ions. Unlike the free amino acid, the histidine residue (i.e., as a component of a peptide or protein), coordinates solely via the imidazole substituent. Examples include myoglobin (Fe), carbonic anhydrase (Zn), azurin (Cu), and alpha-ketoglutarate-dependent hydroxylases Alpha-ketoglutarate-dependent hydroxylases are a major class of non-heme iron proteins that catalyse a wide range of reactions. These reactions include hydroxylation reactions, demethylations, ring expansions, ring closures, and desaturations. Func ...
(Fe). Polyhistidine-tag
A polyhistidine-tag is an amino acid motif in proteins that typically consists of at least six histidine (''His'') residues, often at the N- or C-terminus of the protein. It is also known as hexa histidine-tag, 6xHis-tag, His6 tag, by the US trad ...
("hs tag") is an amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
motif in protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s consisting of several histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
(''His'') residues that is attached to proteins to facilitate purification. The concept relies on the affinity of the imidazole side chain for metal cations.
Reactions of imidazole ligands
Especially in cationic imidazole complexes, the N-H center is acidified. For tricationic d6 pentammines, deprotonation of the imidazole ligand gives imidazolate complexes with pKa near 10 (M = Co, Rh, Ir): : (NH3)5(N2C3H4)sup>3+ (NH3)5(N2C3H3)sup>2+ + H+ The d5 complex u(NH3)5(N2C3H4)sup>3+ is more acidic, with a pKa of 8.9. Thus, complexation to tricationic complexes acidify the pyrrolic NH center by at least 10,000. Imidazole ligands are isomers ofN-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
s. This conversion has been observed:
: u(NH3)5(N2C3H4)sup>2+ → u(NH3)5(C(NH)2(CH)2)sup>2+
Imidazolate complexes
bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually ...
. One example of an imidazolate complex from biochemistry is found at the active site of copper-containing superoxide dismutase.
The M2(μ-imidazolate) motif underpins materials comprising zeolitic imidazolate framework
Zeolitic imidazolate frameworks (ZIFs) are a class of metal-organic frameworks (MOFs) that are topologically isomorphic with zeolites. ZIF glasses can be synthesized by the melt-quench method, and the first melt-quenched ZIF glass was firstly mad ...
s ("ZIF"s).
References