Tolman Electronic Parameter on:
[Wikipedia]
[Google]
[Amazon]
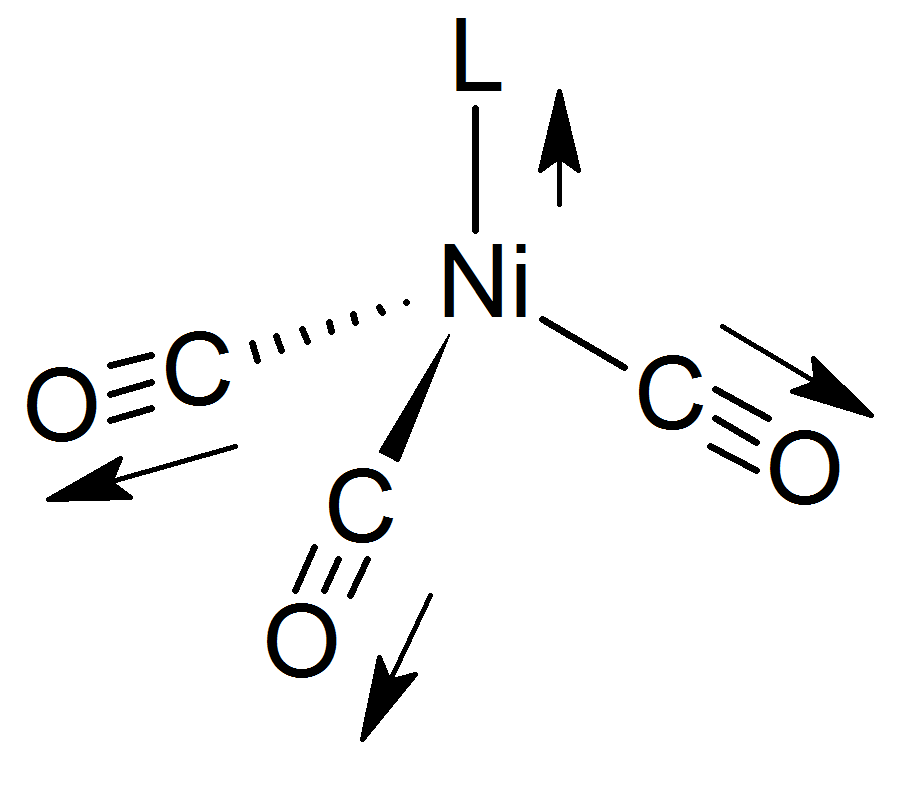 The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a
The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a  The A1 carbonyl band is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis. Upon coordination of CO to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This shift can be explained by
The A1 carbonyl band is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis. Upon coordination of CO to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This shift can be explained by 
 The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a
The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
. It is determined by measuring the frequency of the A1 C-O vibrational mode (ν(CO)) of a (pseudo)-C3v symmetric
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definiti ...
complex, Ni(CO)3by infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, where L is the ligand of interest. Ni(CO)3was chosen as the model compound because such complexes are readily prepared from tetracarbonylnickel(0). The shift in ν(CO) is used to infer the electronic properties of a ligand, which can aid in understanding its behavior in other complexes. The analysis was introduced by Chadwick A. Tolman.
: The A1 carbonyl band is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis. Upon coordination of CO to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This shift can be explained by
The A1 carbonyl band is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis. Upon coordination of CO to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This shift can be explained by π backbonding
In chemistry, π backbonding, also called π backdonation, is when electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a ''π-acceptor ligand''. It is especially common in the organometallic che ...
: the metal forms a π bond with the carbonyl ligand by donating electrons through its d orbitals into the empty π* anti-bonding orbitals on CO. This interaction strengthens the metal-carbon bond but also weakens the carbon-oxygen bond, resulting in a lower vibrational frequency. If other ligands increase the density of π electrons on the metal, the C-O bond is weakened and ν(CO) decreases further; conversely, if other ligands compete with CO for π backbonding, ν(CO) increases.

Other ligand electronic parameters
Several other scales have been proposed for the ranking of the donor properties of ligands. The HEP scale ranks ligands on the basis of the 13C NMR shift of a reference ligand. Lever's electronic parameter ranking is related to the Ru(II/III) couple. Another scale evaluated ligands on the basis of the redox couples of r(CO)5Lsup>0/+. In a treatment akin to the TEP analysis, the donor properties ofN-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
(NHC) ligands have been ranked according to IR data recorded on cis- hCl(NHC)(CO)2complexes.
See also
*Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe ch ...
* Tolman cone angle
In coordination chemistry, the ligand cone angle (a common example being the Tolman cone angle or ''θ'') is a measure of the steric bulk of a ligand in a transition metal coordination complex. It is defined as the solid angle formed with the met ...
References
Further reading
* * {{cite journal , last1 = Gusev , first1 = Dmitry G. , doi = 10.1021/om900654g , title = Electronic and Steric Parameters of 76 N-Heterocyclic Carbenes in Ni(CO)3(NHC) , date = 2009 , pages = 6458–6461 , issue = 22 , volume = 28 , journal =Organometallics
''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is organometallic and organometalloid chemistry. This peer-reviewed journal has an impact factor of 3.837 as reported by the 2021 Journal Citat ...
Organometallic chemistry
Inorganic chemistry
Infrared spectroscopy