Thioformaldehyde on:
[Wikipedia]
[Google]
[Amazon]
Thioformaldehyde is the 
organosulfur compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfu ...
with the formula CH2S. This compound is very rarely observed because it oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
izes to 1,3,5-trithiane, which is a stable colorless compound with the same empirical formula. Despite its instability under normal terrestrial conditions, the molecule has been observed in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
and has attracted much attention for its fundamental nature. The tendency of thioformaldehyde to form chains and rings is a manifestation of the double bond rule
In chemistry, the double bond rule states that elements with a principal quantum number greater than 2 for their valence electrons (period 3 elements and higher) tend not to form multiple bonds (e.g. double bonds and triple bonds). The double bon ...
.
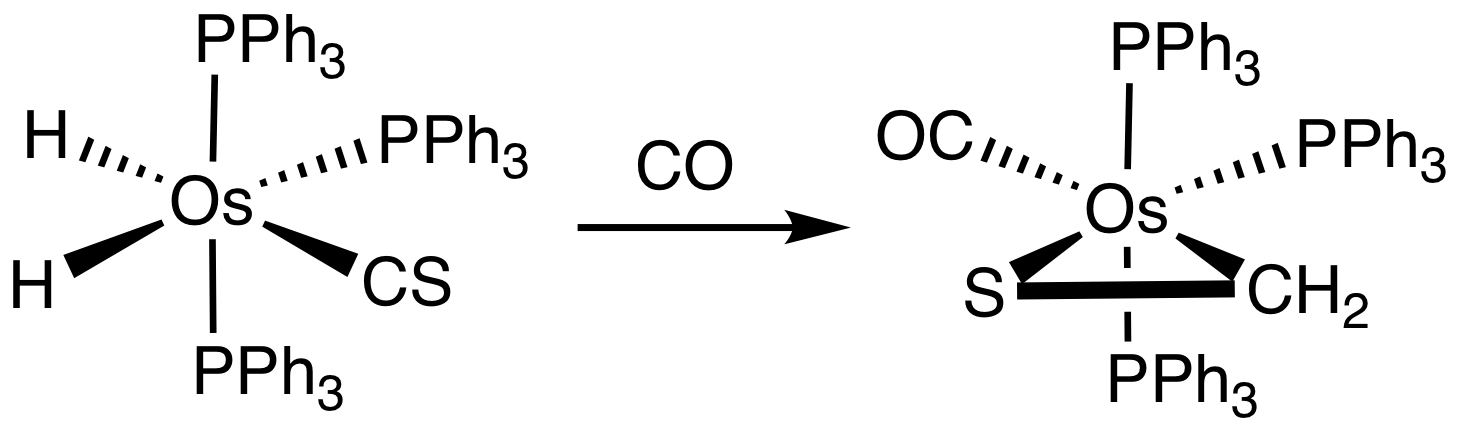
Although thioformaldehyde tends to oligomerize, many metal complexes are known. One example is Os(SCH2)(CO)2(PPh3)2.


References