Thiazyl Fluoride on:
[Wikipedia]
[Google]
[Amazon]
Thiazyl fluoride, NSF, is a colourless, pungent gas at room temperature and condenses to a pale yellow liquid at 0.4 °C. Along with


 Reacting strong Lewis acids, such as AsF5 and SbF5, can cleave NSF to yield the thiazyl cation and a fluoride ion.
By contrast, transition metal cations (which are classified as medium-hard acids) attack at the nitrogen atom due in part to
Reacting strong Lewis acids, such as AsF5 and SbF5, can cleave NSF to yield the thiazyl cation and a fluoride ion.
By contrast, transition metal cations (which are classified as medium-hard acids) attack at the nitrogen atom due in part to  Thiazyl fluoride may be stabilized as a complexing ligand to cationic carbonyl-metal compounds, such as
Thiazyl fluoride may be stabilized as a complexing ligand to cationic carbonyl-metal compounds, such as e(CO)_5NSF .
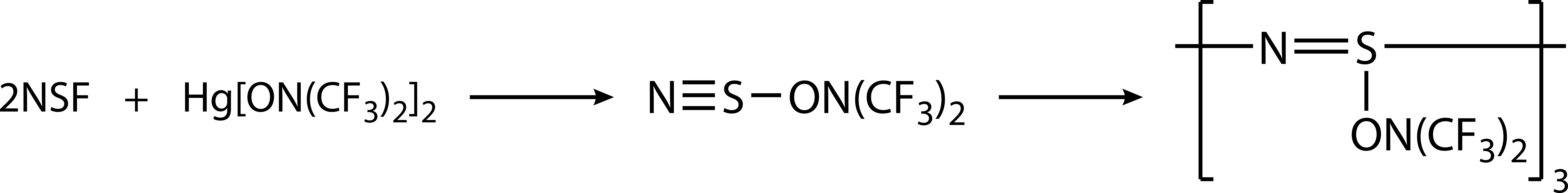 The N≡SON(CF3)2 compound can be stabilized by incorporation of a carbonyl-metal complex.
The N≡SON(CF3)2 compound can be stabilized by incorporation of a carbonyl-metal complex.
 The halogen derivatives XNSF2 (X = F, Cl, Br, I) can be synthesized from reacting Hg(NSF)2 with X2; whereby, ClNSF2 is the most stable compound observed in this series.
The halogen derivatives XNSF2 (X = F, Cl, Br, I) can be synthesized from reacting Hg(NSF)2 with X2; whereby, ClNSF2 is the most stable compound observed in this series.
N-S bond (1.448 Å) is short, indicating multiple bonding, and can be represented by the following resonance structures:
 At room temperature, thiazyl fluoride undergoes cyclic
At room temperature, thiazyl fluoride undergoes cyclic N-S multiple bonding:
 1,3,5-trifluoro-1,3,5,2,4,6-trithiatriazine is the yielded cyclic trimer, where each sulfur atom remains
1,3,5-trifluoro-1,3,5,2,4,6-trithiatriazine is the yielded cyclic trimer, where each sulfur atom remains 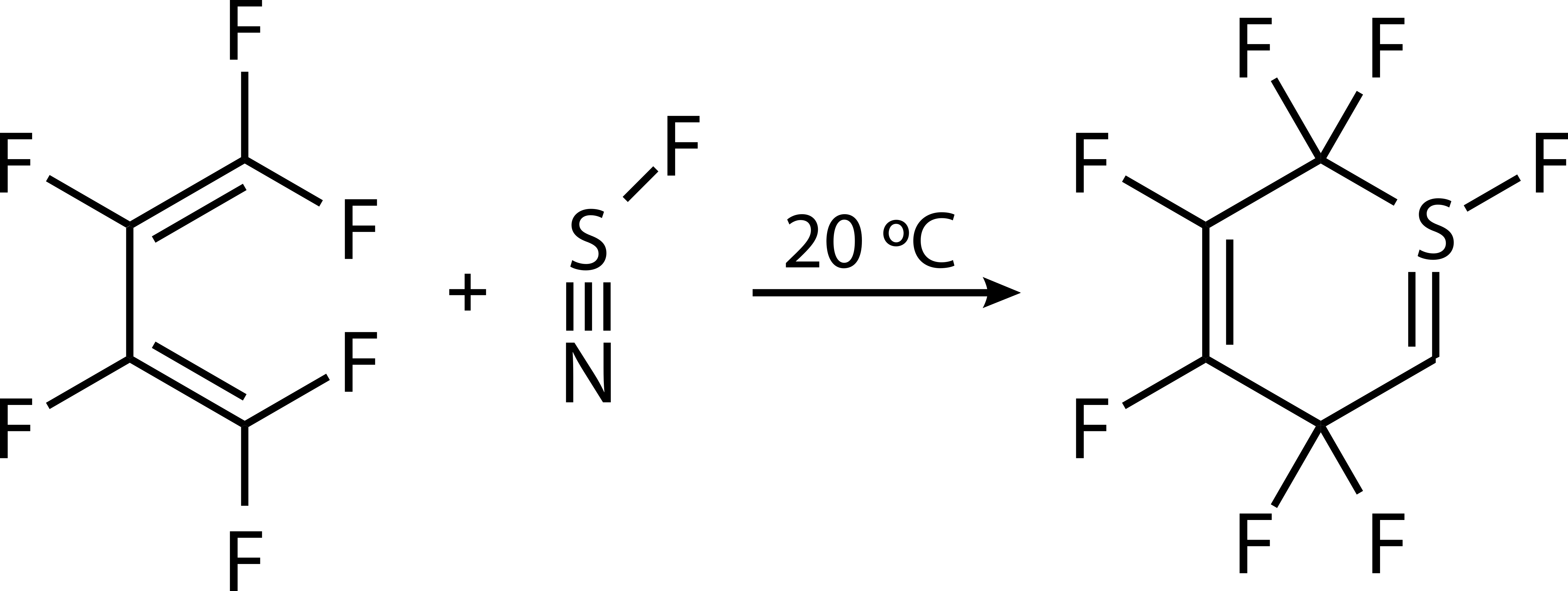

SO_2 . Thiazyl fluoride adopts Cs-symmetry and has been shown by isotopic substitution to be bent in the ground state. A combination of rotational analysis with Franck-Condon calculations has been applied to study the electronic excitation from the A'' A' states, which results in the elongation of the N-S bond by 0.11 Å and a decrease in the NSF by 15.3.
In X-ray structural analysis of the cations (NSF)_6 (M=Co, Ni ), each NSF molecule is bound to the metal center through nitrogen. The observed bond lengths from Co-N to Ni-N are shortened which is related to the contraction of ionic radii as you move across the period.
thiazyl trifluoride
Thiazyl trifluoride is a chemical compound of nitrogen, sulfur, and fluorine, having the formula . It exists as a stable, colourless gas, and is an important precursor to other sulfur-nitrogen-fluorine compounds. It has tetrahedral molecular geome ...
, NSF3, it is an important precursor to sulfur-nitrogen-fluorine compounds. It is notable for its extreme hygroscopicity.
Synthesis
Thiazyl fluoride can be synthesized by various methods, such asfluorination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, ...
of tetrasulfur tetranitride
Tetrasulfur tetranitride is an inorganic compound with the formula . This gold-poppy coloured solid is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N ...
, , with silver(II) fluoride
Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound. Silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.
Preparation
AgF2 can be synthesized by fluori ...
or mercuric fluoride in tetrachloromethane
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemica ...
, and purified by vacuum distillation. However, because this synthetic pathway yields numerous side-products, an alternative approach is the reaction of imino(triphenyl)phosphines with sulfur tetrafluoride by cleavage of the bond to form sulfur difluoride imides and triphenyldifluorophosphorane. These products readily decompose yielding thiazyl fluoride.
For synthesis on a preparative scale, the decomposition of compounds already containing the moiety is commonly used:

Reactivity
Thiazyl fluoride reacts violently with water, decomposes at room temperature, and should be stored at −78 °C.Reactions with Electrophiles
The site of electrophilic attack is primarily dependent upon the strength of theelectrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
. For example, hard Lewis acids
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any spe ...
attack at the site of fluorine to afford thiazyl salts:
Coulombic
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventiona ...
interactions. Soft acids with low valencies may attack at the sulfur atom. In the scheme below, electrophilic attack by a transition metal cation to NSF readily releases sulfur dioxide due to weak σ bonding and minimal π-back bonding at the sulfur atom.
Reactions with Nucleophiles
Nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
attack on thiazyl fluoride occurs at the sulfur atom, whereby either the coordination number of sulfur is increased, or a fluorine atom is substituted.
Nucleophilic Substitution
The triple bond in NSF is retained during nucleophilic substitution, and contributes to trimerization at room temperature.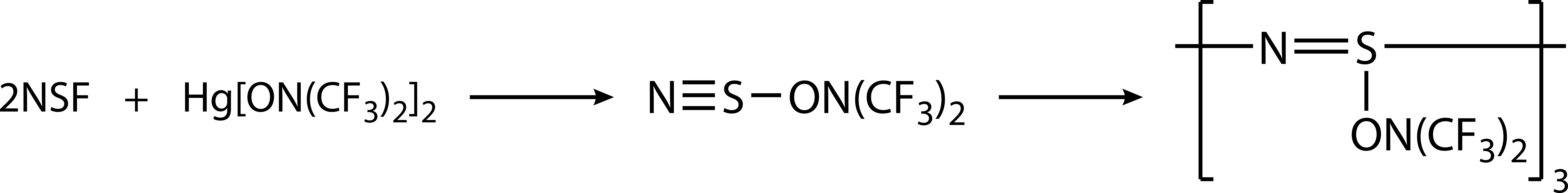 The N≡SON(CF3)2 compound can be stabilized by incorporation of a carbonyl-metal complex.
The N≡SON(CF3)2 compound can be stabilized by incorporation of a carbonyl-metal complex.
Nucleophilic Addition
Nucleophilic attack occurs at the site of the sulfur atom, thereby increasing its coordination number. Reacting cesium fluoride and NSF affords the salt given below. The halogen derivatives XNSF2 (X = F, Cl, Br, I) can be synthesized from reacting Hg(NSF)2 with X2; whereby, ClNSF2 is the most stable compound observed in this series.
The halogen derivatives XNSF2 (X = F, Cl, Br, I) can be synthesized from reacting Hg(NSF)2 with X2; whereby, ClNSF2 is the most stable compound observed in this series.
Oligimerization and Cycloaddition
The length of the At room temperature, thiazyl fluoride undergoes cyclic
At room temperature, thiazyl fluoride undergoes cyclic trimerization
In chemistry, a trimer (; ) is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors ofte ...
via the  1,3,5-trifluoro-1,3,5,2,4,6-trithiatriazine is the yielded cyclic trimer, where each sulfur atom remains
1,3,5-trifluoro-1,3,5,2,4,6-trithiatriazine is the yielded cyclic trimer, where each sulfur atom remains tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
.
Thiazyl fluoride also reacts via exothermic cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
in the presence of dienes.

Properties
Structure and Bonding
The NSF molecule has 18 total valence electrons and is isoelectronic to sulfur dioxide,References
{{reflist Fluorides Nonmetal halides Nitrides Thiohalides Sulfur–nitrogen compounds