Thermal Pressure on:
[Wikipedia]
[Google]
[Amazon]
In
 The thermal pressure coefficient can be considered as a fundamental property; it is closely related to various properties such as
The thermal pressure coefficient can be considered as a fundamental property; it is closely related to various properties such as
 The thermal pressure of a crystal defines how the unit-cell parameters change as a function of
The thermal pressure of a crystal defines how the unit-cell parameters change as a function of
thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
, thermal pressure (also known as the thermal pressure coefficient) is a measure of the relative pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
change of a fluid
In physics, a fluid is a liquid, gas, or other material that continuously deforms (''flows'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are substances which cannot resist any shear ...
or a solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount o ...
as a response to a temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
change at constant volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). The de ...
. The concept is related to the Pressure-Temperature Law, also known as Amontons's law or Gay-Lussac's law
Gay-Lussac's law usually refers to Joseph-Louis Gay-Lussac's law of combining volumes of gases, discovered in 1808 and published in 1809. It sometimes refers to the proportionality of the volume of a gas to its absolute temperature at constant pr ...
.
In general pressure, () can be written as the following sum: .
is the pressure required to compress the material from its volume to volume at a constant temperature . The second term expresses the change in thermal pressure . This is the pressure change at constant volume due to the temperature difference between and . Thus, it is the pressure change along an isochore of the material.
The thermal pressure is customarily expressed in its simple form as
Thermodynamic definition
Because of the equivalences between many properties and derivatives withinthermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
(e.g., see Maxwell Relations
file:Thermodynamic map.svg, 400px, Flow chart showing the paths between the Maxwell relations. P is pressure, T temperature, V volume, S entropy, \alpha coefficient of thermal expansion, \kappa compressibility, C_V heat capacity at constant volu ...
), there are many formulations of the thermal pressure coefficient, which are equally valid, leading to distinct yet correct interpretations of its meaning.
Some formulations for the thermal pressure coefficient include:
Where is the volume thermal expansion
Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions.
Temperature is a monotonic function of the average molecular kinetic ...
, the isothermal bulk modulus
The bulk modulus (K or B) of a substance is a measure of how resistant to compression the substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume.
Other moduli describ ...
, the Grüneisen parameter The Grüneisen parameter, γ, named after Eduard Grüneisen, describes the effect that changing the volume of a crystal lattice has on its vibrational properties, and, as a consequence, the effect that changing temperature has on the size or dynami ...
, the compressibility
In thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility or, if the temperature is held constant, the isothermal compressibility) is a measure of the instantaneous relative volume change of a fl ...
and the constant-volume heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity i ...
. J.M.Haile (2002). "Lectures in Thermodynamics,Volume1".Macatea Productions, Central, South Carolina, 53-67 177-184.
Details of the calculation:
The utility of the thermal pressure
 The thermal pressure coefficient can be considered as a fundamental property; it is closely related to various properties such as
The thermal pressure coefficient can be considered as a fundamental property; it is closely related to various properties such as internal pressure
Internal pressure is a measure of how the internal energy of a system changes when it expands or contracts at constant temperature. It has the same dimensions as pressure, the SI unit of which is the pascal.
Internal pressure is usually given the ...
, sonic velocity
The speed of sound is the distance travelled per unit of time by a sound wave as it propagates through an elastic medium. At , the speed of sound in air is about , or one kilometre in or one mile in . It depends strongly on temperature as ...
, the entropy of melting, isothermal compressibility
In thermodynamics and fluid mechanics, the compressibility (also known as the coefficient of compressibility or, if the temperature is held constant, the isothermal compressibility) is a measure of the instantaneous relative volume change of a f ...
, isobaric expansibility, phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of ...
, etc. Thus, the study of the thermal pressure coefficient provides a useful basis for understanding the nature of liquid and solid. Since it is normally difficult to obtain the properties by thermodynamic and statistical mechanics methods due to complex interactions among molecules, experimental methods attract much attention.
The thermal pressure coefficient is used to calculate results that are applied widely in industry, and they would further accelerate the development of thermodynamic theory.
Commonly the thermal pressure coefficient may be expressed as functions of temperature and volume. There are two main types of calculation of the thermal pressure coefficient: one is the Virial theorem
In mechanics, the virial theorem provides a general equation that relates the average over time of the total kinetic energy of a stable system of discrete particles, bound by potential forces, with that of the total potential energy of the system. ...
and its derivatives; the other is the Van der Waals type and its derivatives. Gilbert Newton Lewi (1900). "A New Conception of Thermal Pressure and a Theory of Solutions". American Academy of Arts & Sciences. .
Thermal pressure at high temperature
As mentioned above, is one of the most common formulations for the thermal pressure coefficient. Both and are affected by temperature changes, but the value of and of a solid much less sensitive to temperature change above itsDebye temperature
In thermodynamics and solid-state physics, the Debye model is a method developed by Peter Debye in 1912 for estimating the phonon contribution to the specific heat (Heat capacity) in a solid. It treats the vibrations of the atomic lattice (hea ...
. Thus, the thermal pressure of a solid due to moderate temperature change above the Debye temperature can be approximated by assuming a constant value of and . Angel, Ross J., Miozzi Francesca, and Alvaro Matteo (2019). "Limits to the Validity of Thermal-Pressure Equations of State". MDPI.
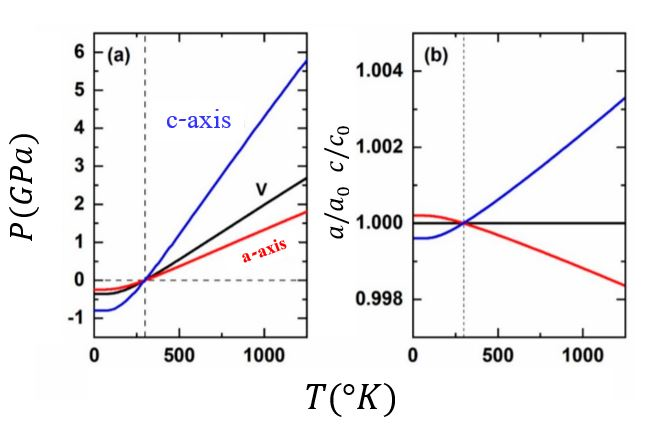
Thermal pressure in a crystal
 The thermal pressure of a crystal defines how the unit-cell parameters change as a function of
The thermal pressure of a crystal defines how the unit-cell parameters change as a function of pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
and temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
. Therefore, it also controls how the cell parameters change along an isochore, namely as a function of . Usually, Mie-Grüneisen-Debye and other Quasi harmonic approximation (QHA) based state functions are being used to estimate volumes and densities of mineral phases in diverse applications such as thermodynamic, deep-Earth geophysical models and other planetary bodies. In the case of isotropic (or approximately isotropic) thermal pressure, the unit cell parameter remains constant along the isochore and the QHA is valid. But when the thermal pressure is anisotropic, the unit cell parameter changes so, the frequencies of vibrational modes also change even in constant volume and the QHA is no longer valid.
The combined effect of a change in pressure and temperature is described by the strain tensor
In continuum mechanics, the infinitesimal strain theory is a mathematical approach to the description of the deformation of a solid body in which the displacements of the material particles are assumed to be much smaller (indeed, infinitesimally ...
:
Where is the volume thermal expansion tensor and is the compressibility tensor. The line in the P-T space which indicates that the strain is constant in a particular direction within the crystal is defined as:
Which is an equivalent definition of the isotropic degree of thermal pressure.
See also
*Isochoric process
In thermodynamics, an isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant ...
* Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
* Hydrostatic equilibrium
In fluid mechanics, hydrostatic equilibrium (hydrostatic balance, hydrostasy) is the condition of a fluid or plastic solid at rest, which occurs when external forces, such as gravity, are balanced by a pressure-gradient force. In the planetary ...
References
Thermodynamics {{Improve categories, date=September 2020