Thermal desorption spectroscopy on:
[Wikipedia]
[Google]
[Amazon]
Temperature programmed desorption (TPD) is the method of observing
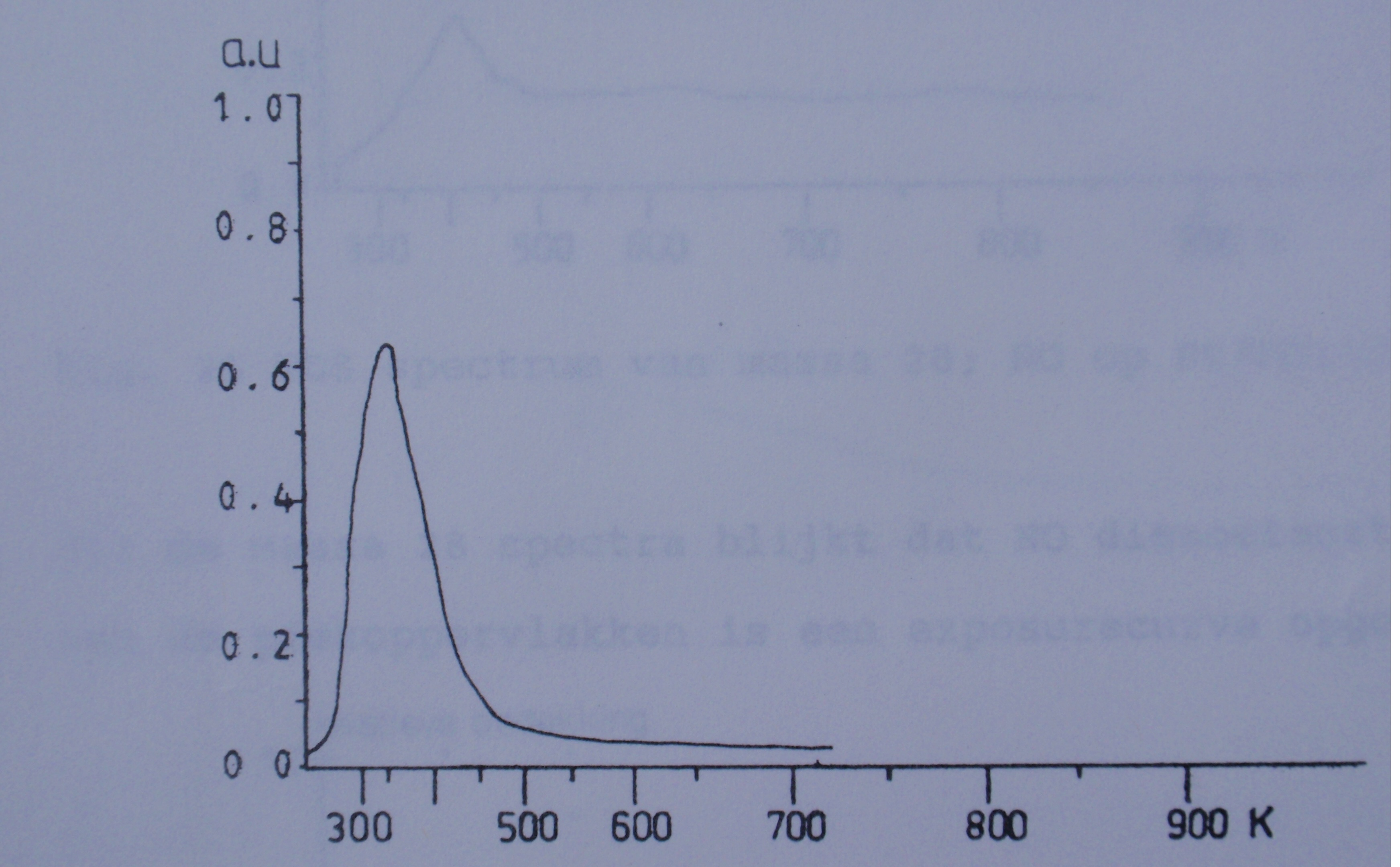 TDS spectrum 1 and 2 are typical examples of a TPD measurement. Both are examples of NO desorbing from a single crystal in high vacuum. The crystal was mounted on a titanium filament and heated with current. The desorbing NO was measured using a mass spectrometer monitoring the atomic mass of 30.
Before 1990 analysis of a TPD spectrum was usually done using a so-called simplified method; the "Redhead" method, assuming the exponential prefactor and the desorption energy to be independent of the surface coverage. After 1990 and with use of computer algorithms TDS spectra were analyzed using the "complete analysis method" or the "leading edge method". These methods assume the exponential prefactor and the desorption energy to be dependent of the surface coverage. Several available methods of analyzing TDS are described and compared in an article by A.M. de JONG and J.W. NIEMANTSVERDRIET. During parameter optimization/estimation, using the integral has been found to create a more well behaved objective function than the differential.
TDS spectrum 1 and 2 are typical examples of a TPD measurement. Both are examples of NO desorbing from a single crystal in high vacuum. The crystal was mounted on a titanium filament and heated with current. The desorbing NO was measured using a mass spectrometer monitoring the atomic mass of 30.
Before 1990 analysis of a TPD spectrum was usually done using a so-called simplified method; the "Redhead" method, assuming the exponential prefactor and the desorption energy to be independent of the surface coverage. After 1990 and with use of computer algorithms TDS spectra were analyzed using the "complete analysis method" or the "leading edge method". These methods assume the exponential prefactor and the desorption energy to be dependent of the surface coverage. Several available methods of analyzing TDS are described and compared in an article by A.M. de JONG and J.W. NIEMANTSVERDRIET. During parameter optimization/estimation, using the integral has been found to create a more well behaved objective function than the differential.
(equation 1) : where: : the heating rate in /s : the start temperature in : the time in We assume that the pump rate of the system is indefinitely large, thus no gasses will absorb during the desorption. The change in pressure during desorption is described as:
(equation 2) : where: : the pressure in the system, : the time in :, : the sample surface 2 : a constant, : volume of the system 3 : the desorption rate ol/(cm2 s) :, : the pump rate, : volume of the system 3 We assume that is indefinitely large so molecules do not re-adsorp during desorption process and we assume that is indefinitely small compared to and thus:
(equation 3) : Equation 2 and 3 lead to conclude that the desorption rate is a function of the change in pressure. One can use data in an experiment, which are a function of the pressure like the intensity of a mass spectrometer, to determine the desorption rate. Since we assumed the pre-exponential factor and the activation energy to be independent of the coverage. Thermal desorption is described with a simplified
(equation 4) : where: : the desorption rate ol/(cm2 s) : order of desorption, : surface coverage, : pre-exponential factor z : activation energy of desorption J/mol :
(equation 5)
for n=1 :
(equation 6)
for n=2 : M. Ehasi and K. Christmann described a simple method to determine the activation energy of the second order. Equation 6 can be changed into:
(equation 6a) : where: is the surface area of a TDS or TPD peak. A graph of versus results in a straight line with a slope equal to . Thus in a first-order reaction the is independent of the surface coverage. Changing the surface coverage one can determine . Usually a fixed value of the pre-exponential factor is used and is known, with these values one can derive the iteratively from .
Temperature programmed desorption
@ the Surface Science Laboratory
{{DEFAULTSORT:Thermal Programmed Desorption Mass spectrometry Analytical chemistry Surface science
desorbed
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.
There ...
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s from a surface
A surface, as the term is most generally used, is the outermost or uppermost layer of a physical object or space. It is the portion or region of the object that can first be perceived by an observer using the senses of sight and touch, and is t ...
when the surface temperature is increased. When experiments are performed using well-defined surfaces of single-crystalline samples in a continuously pumped ultra-high vacuum (UHV) chamber, then this experimental technique is often also referred to as thermal desorption spectroscopy or thermal desorption spectrometry (TDS).
Desorption
When molecules or atoms come in contact with a surface, theyadsorb
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fl ...
onto it, minimizing their energy by forming a bond with the surface. The binding energy varies with the combination of the adsorbate and surface. If the surface is heated, at one point, the energy transferred to the adsorbed species will cause it to desorb. The temperature at which this happens is known as the desorption temperature. Thus TPD shows information on the binding energy.
Measurement
Since TPD observes the mass of desorbed molecules, it shows what molecules are adsorbed on the surface. Moreover, TPD recognizes the different adsorption conditions of the same molecule from the differences between the desorption temperatures of molecules desorbing different sites at the surface, e.g. terraces vs. steps. TPD also obtains the amounts of adsorbed molecules on the surface from the intensity of the peaks of the TPD spectrum, and the total amount of adsorbed species is shown by the integral of the spectrum. To measure TPD, one needs a mass spectrometer, such as aquadrupole mass spectrometer
The quadrupole mass analyzer, originally conceived by Nobel Laureate Wolfgang Paul and his student Helmut Steinwedel, also known as quadrupole mass filter, is one type of mass analyzer used in mass spectrometry. As the name implies, it consists of ...
or a time-of-flight (TOF) mass spectrometer, under ultrahigh vacuum (UHV) conditions. The amount of adsorbed molecules is measured by increasing the temperature at a heating rate of typically 2 K/s to 10 K/s. Several masses may be simultaneously measured by the mass spectrometer, and the intensity of each mass as a function of temperature is obtained as a TDS spectrum.
The heating procedure is often controlled by the PID control
A proportional–integral–derivative controller (PID controller or three-term controller) is a control loop mechanism employing feedback that is widely used in industrial control systems and a variety of other applications requiring continuous ...
algorithm, with the controller being either a computer or specialised equipment such as a Eurotherm
Eurotherm is a supplier of control and measurement instruments to industrial and process markets. They are part of Watlow, an electricity distribution, automation management and producer of installation components for energy management company. E ...
.
Other methods of measuring desorption are Thermal Gravimetric Analysis (TGA) or using infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
detectors, thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
detectors etc.
Quantitative interpretation of TPD data
Theoretical Introduction
Thermal desorption is described based on theArrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 18 ...
.
:
where
: the desorption rate ol/(cm2 s)as a function of ,
: order of desorption,
: surface coverage,
: pre-exponential factor zas a function of ,
: activation energy of desorption J/molas a function of ,
: gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
/(K mol)
: temperature
This equation is difficult in practice while several variables are a function of the coverage and influence each other. The “complete analysis method” calculates the pre-exponential factor and the activation energy at several coverages. This calculation can be simplified. First we assume the pre-exponential factor and the activation energy to be independent of the coverage.
We also assume a linear heating rate:
(equation 1) : where: : the heating rate in /s : the start temperature in : the time in We assume that the pump rate of the system is indefinitely large, thus no gasses will absorb during the desorption. The change in pressure during desorption is described as:
(equation 2) : where: : the pressure in the system, : the time in :, : the sample surface 2 : a constant, : volume of the system 3 : the desorption rate ol/(cm2 s) :, : the pump rate, : volume of the system 3 We assume that is indefinitely large so molecules do not re-adsorp during desorption process and we assume that is indefinitely small compared to and thus:
(equation 3) : Equation 2 and 3 lead to conclude that the desorption rate is a function of the change in pressure. One can use data in an experiment, which are a function of the pressure like the intensity of a mass spectrometer, to determine the desorption rate. Since we assumed the pre-exponential factor and the activation energy to be independent of the coverage. Thermal desorption is described with a simplified
Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 18 ...
:
(equation 4) : where: : the desorption rate ol/(cm2 s) : order of desorption, : surface coverage, : pre-exponential factor z : activation energy of desorption J/mol :
gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
,
: temperature
Using the before mentioned Redhead method (a method less precise as the "complete analysis" or the "leading edge" method) and the temperature maximum one can determine the activation energy:
(equation 5)
for n=1 :
(equation 6)
for n=2 : M. Ehasi and K. Christmann described a simple method to determine the activation energy of the second order. Equation 6 can be changed into:
(equation 6a) : where: is the surface area of a TDS or TPD peak. A graph of versus results in a straight line with a slope equal to . Thus in a first-order reaction the is independent of the surface coverage. Changing the surface coverage one can determine . Usually a fixed value of the pre-exponential factor is used and is known, with these values one can derive the iteratively from .
See also
*Temperature-programmed reduction Temperature-programmed reduction (TPR) is a technique for the characterization of solid materials and is often used in the field of heterogeneous catalysis to find the most efficient reduction conditions, an oxidized catalyst precursor is submitted ...
References
External links
Temperature programmed desorption
@ the Surface Science Laboratory
{{DEFAULTSORT:Thermal Programmed Desorption Mass spectrometry Analytical chemistry Surface science