Tetrasulfur on:
[Wikipedia]
[Google]
[Amazon]
 The element
The element
 The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
 This allotrope was first prepared by M. R. Engel in 1891 by treating
This allotrope was first prepared by M. R. Engel in 1891 by treating
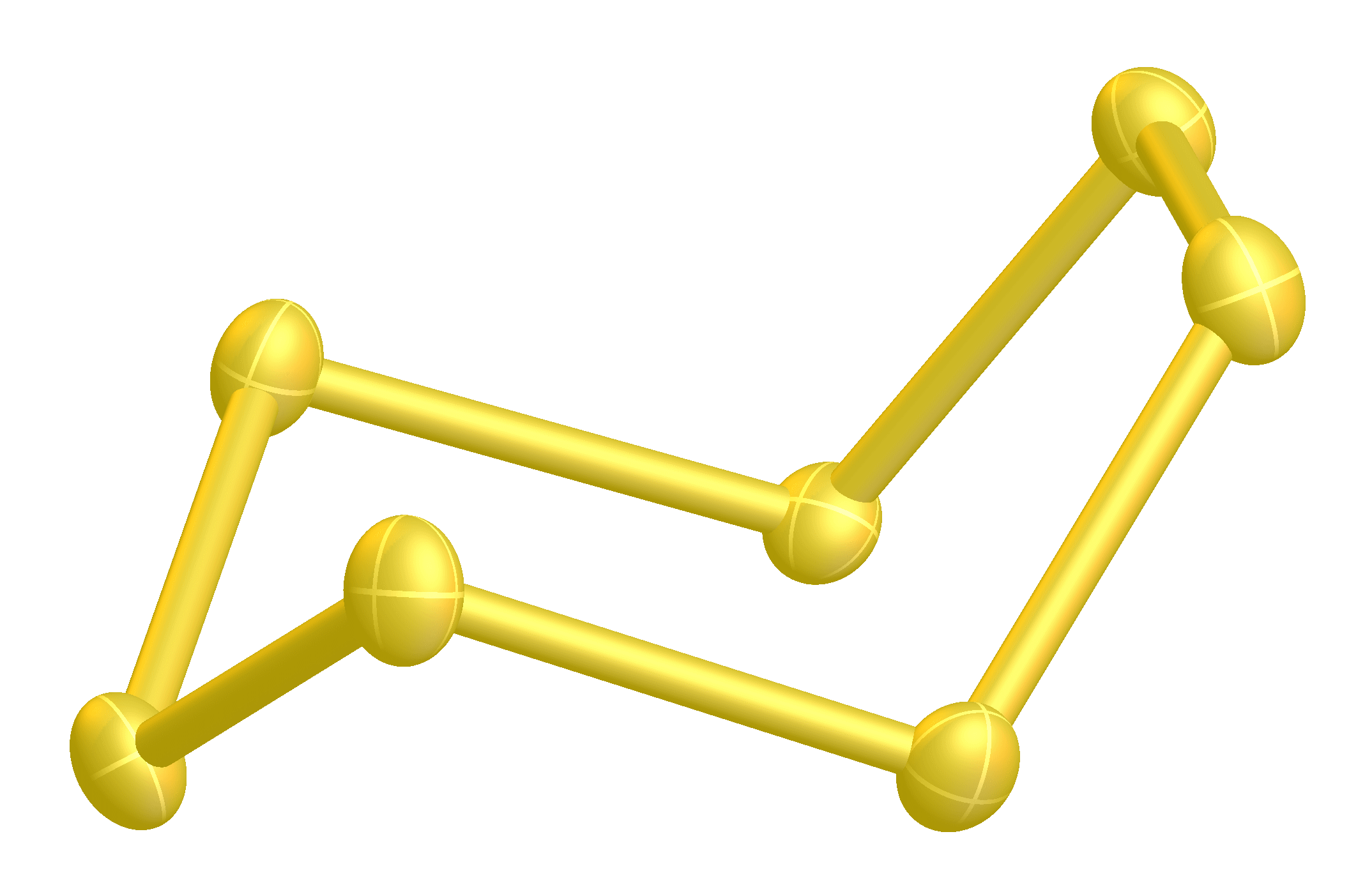 It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known.
It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known.
 These allotropes have been synthesised by various methods for example, treating
These allotropes have been synthesised by various methods for example, treating
 The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
 The element
The element sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
exists as many allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
. In number of allotropes, sulfur is second only to carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 652 In addition to the allotropes, each allotrope often exists in polymorphs (different crystal structures of the same covalently bonded S molecules) delineated by Greek prefixes (α, β, etc.).
Furthermore, because elemental sulfur has been an item of commerce for centuries, its various forms are given traditional names. Early workers identified some forms that have later proved to be single or mixtures of allotropes. Some forms have been named for their appearance, e.g. "mother of pearl sulfur", or alternatively named for a chemist who was pre-eminent in identifying them, e.g. "Muthmann's sulfur I" or "Engel's sulfur".Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 17
The most commonly encountered form of sulfur is the orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
polymorph of , which adopts a puckered ring – or "crown" – structure. Two other polymorphs are known, also with nearly identical molecular structures.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 654 In addition to S8, sulfur rings of 6, 7, 9–15, 18, and 20 atoms are known.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 655 At least five allotropes are uniquely formed at high pressures, two of which are metallic.
The number of sulfur allotropes reflects the relatively strong S−S bond of 265 kJ/mol. Furthermore, unlike most elements, the allotropes of sulfur can be manipulated in solutions of organic solvents and are amenable to analysis by HPLC
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa ...
.
Phase diagram
 The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
The pressure-temperature (P-T) phase diagram for sulfur is complex (see image). The region labeled I (a solid region), is α-sulfur.
High pressure solid allotropes
In a high-pressure study at ambient temperatures, four new solid forms, termed II, III, IV, V have been characterized, where α-sulfur is form I. Solid forms II and III are polymeric, while IV and V are metallic (and aresuperconductive
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
below 10 K and 17 K, respectively). Laser irradiation of solid samples produces three sulfur forms below 200–300 kbar (20–30 GPa).
Solid ''cyclo'' allotrope preparation
Two methods exist for the preparation of the ''cyclo''-sulfur allotropes. One of the methods, which is most famous for preparing hexasulfur, is to treat hydrogen polysulfides with polysulfur dichloride: : H2S''x'' + S''y''Cl2 → ''cyclo''-S''x+y'' + 2 HCl A second strategy usestitanocene pentasulfide
Titanocene pentasulfide is the organotitanium compound with the formula (C5H5)2TiS5, commonly abbreviated as Cp2TiS5. This metallocene exists as a bright red solid that is soluble in organic solvents. It is of academic interest as a precursor to u ...
as a source of the S52− unit. This complex is easily made from polysulfide solutions:
: H4sub>2 5+ ( η5-C5H5)2TiCl2 → (C5H5)2TiS5 + 2 NH4Cl
Titanocene pentasulfide reacts with polysulfur chloride:
: ( η5-C5H5)2TiS5 + S''y''Cl2 → ''cyclo''-S''y''+5 + ( η5-C5H5)2TiCl2
Solid cyclo-sulfur allotropes
''Cyclo''-hexasulfur, ''cyclo''-S6
 This allotrope was first prepared by M. R. Engel in 1891 by treating
This allotrope was first prepared by M. R. Engel in 1891 by treating thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
with HCl. Cyclo-S6 is orange-red and forms a rhombohedral crystal.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 656 It is called ρ-sulfur, ε-sulfur, Engel's sulfur and Aten's sulfur. Another method of preparation involves the reaction of a polysulfane
A polysulfane is a chemical compound of formula , where ''n'' > 1 (although disulfane () is sometimes excluded). Polysulfanes consist of unbranched chains of sulfur atoms terminated with hydrogen atoms. Compounds containing 2 – 8 concatenated s ...
with sulfur monochloride
Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S2Cl2.
Some alternative names for this compound are ''sulfur monochloride'' (the name implied by its empirical formula, SCl), ''disulphur dichloride'' (British ...
:
:H2S4 + S2Cl2 → cyclo-S6 + 2 HCl (dilute solution in diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
)
The sulfur ring in cyclo-S6 has a "chair" conformation, reminiscent of the chair form of cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
. All of the sulfur atoms are equivalent.
''Cyclo''-heptasulfur, ''cyclo''-S7
 It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known.
It is a bright yellow solid. Four (α-, β-, γ-, δ-) forms of cyclo-heptasulfur are known.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 657 Two forms (γ-, δ-)have been characterized. The cyclo-S7 ring has an unusual range of bond lengths of 199.3–218.1 pm. It is said to be the least stable of all of the sulfur allotropes.Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 6
''Cyclo''-octasulfur, ''cyclo''-S8
α-Sulfur
α-Sulfur is the form most commonly found in nature. When pure it has a greenish-yellow colour (traces of cyclo-S7 in commercially available samples make it appear yellower). It is practically insoluble in water and is a good electrical insulator with poor thermal conductivity. It is quite soluble in carbon disulfide: 35.5 g/100 g solvent at 25 °C. It has an orthorhombic crystal structure. α-Sulfur is the predominant form found in "flowers of sulfur", "roll sulfur" and "milk of sulfur". It contains S8 puckered rings, alternatively called a crown shape. The S–S bond lengths are all 203.7 pm and the S-S-S angles are 107.8° with a dihedral angle of 98°. At 95.3 °C, α-sulfur converts to β-sulfur.β-Sulfur
β-Sulfur is a yellow solid with a monoclinic crystal form and is less dense than α-sulfur. Like the α- form it contains puckered S8 rings and only differs from it in the way the rings are packed in the crystal. It is unusual because it is only stable above 95.3 °C; below this temperature it converts to α-sulfur. β-Sulfur can be prepared by crystallising at 100 °C and cooling rapidly to slow down formation of α-sulfur. It has a melting point variously quoted as 119.6 °C and 119.8 °C but as it decomposes to other forms at around this temperature the observed melting point can vary. The 119 °C melting point has been termed the "ideal melting point" and the typical lower value (114.5 °C) when decomposition occurs, the "natural melting point".γ-Sulfur
γ-Sulfur was first prepared by F.W. Muthmann in 1890. It is sometimes called "nacreous sulfur" or "mother of pearl sulfur" because of its appearance. It crystallises in pale yellow monoclinic needles. It contains puckered S8 rings like α-sulfur and β-sulfur and only differs from them in the way that these rings are packed. It is the densest form of the three. It can be prepared by slowly cooling molten sulfur that has been heated above 150 °C or by chilling solutions of sulfur in carbon disulfide, ethyl alcohol orhydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
s. It is found in nature as the mineral rosickyite.Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 7 It has been tested in carbon fiber-stabilized form as a cathode in lithium-sulfur (Li-S) batteries and was observed to stop the formation of polysulfides that compromise battery life.
''Cyclo''-Sn (n = 9–15, 18, 20)
 These allotropes have been synthesised by various methods for example, treating
These allotropes have been synthesised by various methods for example, treating titanocene pentasulfide
Titanocene pentasulfide is the organotitanium compound with the formula (C5H5)2TiS5, commonly abbreviated as Cp2TiS5. This metallocene exists as a bright red solid that is soluble in organic solvents. It is of academic interest as a precursor to u ...
and a dichlorosulfane
Sulfur dichloride is the chemical compound with the formula . This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance ...
of suitable sulfur chain length, S''n''−5Cl2:
:( η5-C5H5)2TiS5 + S''n''−5Cl2 → cyclo-S''n''+( η5-C5H5)2TiCl2
or alternatively treating a dichlorosulfane
Sulfur dichloride is the chemical compound with the formula . This cherry-red liquid is the simplest sulfur chloride and one of the most common, and it is used as a precursor to organosulfur compounds. It is a highly corrosive and toxic substance ...
, S''n''−''m''Cl2 and a polysulfane
A polysulfane is a chemical compound of formula , where ''n'' > 1 (although disulfane () is sometimes excluded). Polysulfanes consist of unbranched chains of sulfur atoms terminated with hydrogen atoms. Compounds containing 2 – 8 concatenated s ...
, H2S''m'':
:S''n''−''m''Cl2 + H2S''m'' → cyclo-S''n''+2 HCl
S12, S18, and S20 can also be prepared from S8. With the exception of cyclo-S12, the rings contain S–S bond lengths and S-S-S bond angle that differ one from another.
Cyclo-S12 is the most stable cyclo-allotrope. Its structure can be visualised as having sulfur atoms in three parallel planes, 3 in the top, 6 in the middle and three in the bottom.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 658
Two forms (α-, β-) of cyclo-S9 are known, one of which has been characterized.
Two forms of cyclo-S18 are known where the conformation of the ring is different. To differentiate these structures, rather than using the normal crystallographic convention of α-, β-, etc., which in other cyclo-S''n'' compounds refer to different packings of essentially the same conformer, these two conformers
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a molec ...
have been termed endo- and exo-.
''Cyclo''-S6.''cyclo''-S10 adduct
This adduct is produced from a solution of cyclo-S6 and cyclo-S10 in CS2. It has a density midway between cyclo-S6 and cyclo-S10. The crystal consists of alternate layers of cyclo-S6 and cyclo-S10. This material is a rare example of an allotrope that contains molecules of different sizes.Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 9
Catena sulfur forms
''Catena sulfur forms'' refers to mixtures of sulfur allotropes that are high incatena
Catena (Latin for chain) or catenae (plural) may refer to:
Science
* ''Catena'' (fly), a genus in the family Tachinidae
*Catena (linguistics) is a unit of syntax and morphology, closely associated with dependency grammars
* Catena (computing), nu ...
(polymer chain) sulfur. The naming of the different forms is very confusing and care has to be taken to determine what is being described because some names are used interchangeably.
Amorphous sulfur
Amorphous sulfur is the quenched product from molten sulfur hotter than the λ-transition at 160 °C, where polymerization yields catena sulfur molecules. (Above this temperature, the properties of the liquid melt change remarkably. For example, the viscosity increases more than 10000-fold as the temperature increases through the transition). As it anneals, solid amorphous sulfur changes from its initial glassy form, to a plastic form, hence its other names of ''plastic'', and ''glassy'' or ''vitreous'' sulfur. The plastic form is also called χ-sulfur. Amorphous sulfur contains a complex mixture of catena-sulfur forms mixed with cyclo-forms.Insoluble sulfur
Insoluble sulfur is obtained by washing quenched liquid sulfur with CS2.Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 3 It is sometimes called polymeric sulfur, μ-S or ω-S.
Fibrous (φ-) sulfur
Fibrous (φ-) sulfur is a mixture of the allotropic ψ- form and γ-cycloS8.Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 43
ω-Sulfur
ω-Sulfur is a commercially available product prepared from amorphous sulfur that has not been stretched prior to extraction of soluble forms with CS2. It sometimes called "white sulfur of Das" or supersublimated sulfur. It is a mixture of ψ-sulfur and lamina sulfur. The composition depends on the exact method of production and the sample's history. One well known commercial form is "Crystex". ω-sulfur is used in the vulcanization of rubber.Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 15
λ-Sulfur
λ-Sulfur is molten sulfur just above the melting temperature. It is a mixture containing mostly ''cyclo''-S8. Cooling λ-sulfur slowly gives predominantly β-sulfur.Steudel Steudel is a surname
In some cultures, a surname, family name, or last name is the portion of one's personal name that indicates one's family, tribe or community.
Practices vary by culture. The family name may be placed at either the start of ...
, 26
μ-Sulfur
μ-Sulfur is the name applied to solid insoluble sulfur and the melt prior to quenching.π-Sulfur
π-Sulfur is a dark-coloured liquid formed when λ-sulfur is left to stay molten. It contains mixture of Sn rings.Biradical catena (S∞) chains
This term is applied to biradical catena- chains in sulfur melts or the chains in the solid.Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 662
Solid catena allotropes
 The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
The production of pure forms of catena-sulfur has proved to be extremely difficult. Complicating factors include the purity of the starting material and the thermal history of the sample.
ψ-Sulfur
This form, also called fibrous sulfur or ω1-sulfur, has been well characterized. It has a density of 2.01 g·cm−3 (α-sulfur 2.069 g·cm−3) and decomposes around its melting point of 104 °C. It consists of parallel helical sulfur chains. These chains have both left and right-handed "twists" and a radius of 95 pm. The S–S bond length is 206.6 pm, the S-S-S bond angle is 106° and the dihedral angle is 85.3°, (comparable figures for α-sulfur are 203.7 pm, 107.8° and 98.3°).Greenwood Green wood is unseasoned wood.
Greenwood or Green wood may also refer to:
People
* Greenwood (surname)
Settlements
Australia
* Greenwood, Queensland, a locality in the Toowoomba Region
* Greenwood, Western Australia, a suburb of Perth
C ...
, 660
Lamina sulfur
Lamina sulfur has not been well characterized but is believed to consist of criss-crossed helices. It is also called χ-sulfur or ω2-sulfur.High-temperature gaseous allotropes
Disulfur, S2
Disulfur, S2, is the predominant species in sulfur vapour above 720 °C (a temperature above that shown in the phase diagram); at low pressure (1 mmHg) at 530 °C, it comprises 99% of the vapor. It is a triplet diradical (like dioxygen and sulfur monoxide), with an S−S bond length of 188.7 pm. The blue colour of burning sulfur is due to the emission of light by the S2 molecule produced in the flame. The S2 molecule has been trapped in the compound 2I4EF6]2 (E = Arsenic, As, Sb) for crystallographic measurements, produced by treating elementalsulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
with excess iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
in liquid sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
. The 2I4sup>2+ cation has an "open-book" structure, in which each 2sup>+ ion donates the unpaired electron in the π* molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
to a vacant orbital of the S2 molecule.
Trisulfur, S3
S3 is found in sulfur vapour, comprising 10% of vapour species at 440 °C and 10 mmHg. It is cherry red in colour, with a bent structure, similar toozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
, O3.
Tetrasulfur, S4
S4 has been detected in the vapour phase, but it has not been well characterized. Diverse structures (e.g. chains, branched chains and rings) have been proposed. Theoretical calculations suggest a cyclic structure.Pentasulfur, S5
Pentasulfur has been detected in sulfur vapours but has not been isolated in pure form.List of allotropes and forms
Allotropes are in Bold.References
Bibliography
* *External links
* {{DEFAULTSORT:Sulfur allotropes Amorphous solids