Tetraphenylporphyrin on:
[Wikipedia]
[Google]
[Amazon]
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic
File:PicketFenceGenericRevised.png, A picket-fence porphyrin complex of Fe, with axial coordination sites occupied by methylimidazole (green) and
Sulfonated derivatives of TPP are also well known to give water-soluble derivatives, e.g. tetraphenylporphine sulfonate:
:4 SO3 + (C6H5C)4(C4H2N)2(C4H2NH)2
→ (HO3SC6H4C)4(C4H2N)2(C4H2NH)2 + 4 H2O
 H2TPP is a
H2TPP is a
heterocyclic compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
that resembles naturally occurring porphyrin
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical com ...
s. Porphyrins are dyes and cofactors found in hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocyte ...
and cytochrome
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of bi ...
s and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to ...
and benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
.
Synthesis and structure
Tetraphenylporphyrin was first synthesized in 1935 by Rothemund, who caused benzaldehyde and pyrrole to react in a sealed bomb at 150 °C for 24 h. Adler and Longo modified the Rothemund method by allowing benzaldehyde and pyrrole to react for 30 min in refluxing propionic acid (141 °C) open to the air: :8 C4H4NH + 8 C6H5CHO + 3 O2 → 2 (C6H5C)4(C4H2N)2(C4H2NH)2 + 14 H2O Despite its modest yields, the synthesis of H2TPP is a common experiment in university teaching labs. Highly efficient routes to H2TPP and many analogues involve the air-free condensation of the pyrrole and aldehyde to give theporphyrinogen
In biochemistry a porphyrinogen is a member of a class of naturally occurring compounds with a tetrapyrrole core, a macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples in ...
. In this so-called Lindsey synthesis of meso-substituted porphyrins, the porphyrinogen is subsequently oxidized to deliver the porphyrin.
The conjugate base of the porphyrin, TPP2−, belongs to the symmetry group D4h while its hydrogenated counterpart H2(TPP) is D2h. Unlike natural porphyrins, H2TPP is substituted at the oxidatively sensitive "meso" carbon positions, and hence the compound is sometimes called ''meso''-tetraphenylporphyrin. Another synthetic porphyrin, octaethylporphyrin
Octaethylporphyrin (H2OEP) is an organic compound that is a relative of naturally occurring heme pigments. The compound is used in the preparation of models for the prosthetic group in heme proteins. It is a dark purple solid that is soluble in ...
(H2OEP) does have a substitution pattern that is biomimetic. Many derivatives of TPP and OEP are known, including those prepared from substituted benzaldehydes. One of the first functional analogues of myoglobin was the ferrous derivative of the "picket fence porphyrin," which is structurally related to Fe(TPP), being derived via the condensation of 2-nitrobenzaldehyde and pyrrole.
dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
* ...
(R = amide groups).
File:FeTPP(CCl2)Mansuy.png, Structure of Fe(TPP)CC(C6H4Cl)2, one of several iron carbenoid complexes reported by Daniel Mansuy.
Complexes
Complexation can be thought of as proceeding via the conversion of H2TPP to TPP2−, with 4-fold symmetry. The metal insertion process proceeds via several steps, not via the dianion. Representative complexes: *Cu(TPP) *Zn(TPP)Lx *VO(TPP) * Fe(TPP)ClOptical properties
Tetraphenylporphyrin has a strong absorption band with maximum at 419 nm (so called Soret band) and four weak bands with maxima at 515, 550, 593 and 649 nm (so called Q-bands). It shows red fluorescence with maxima at 649 and 717 nm. The quantum yield is 11%. Soret red shifts for Zn(TTP)-Donor systems relative to the Soret band at 416.2 nm for Zn(TTP) in cyclohexane have been measured.Applications
 H2TPP is a
H2TPP is a photosensitizer
Photosensitizers produce a physicochemical change in a neighboring molecule by either donating an electron to the substrate or by abstracting a hydrogen atom from the substrate. At the end of this process, the photosensitizer eventually returns t ...
for the production of singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
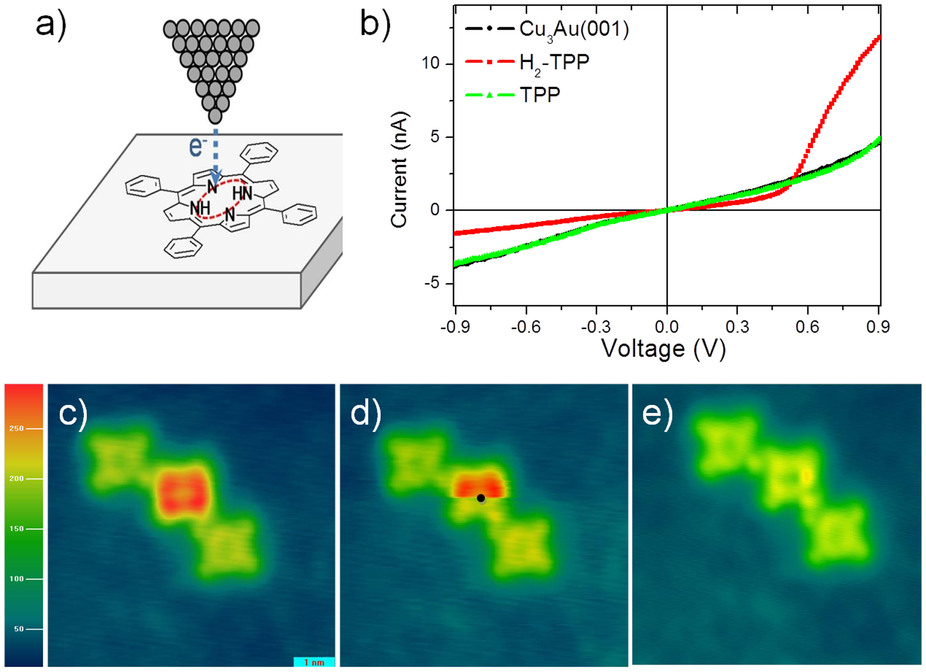
.Karl-Heinz Pfoertner (2002) "Photochemistry" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. Its molecules have potential applications in single-molecule electronics, as they show diode-like behavior that can be altered for each individual molecule.
References
{{Reflist Chelating agents Tetrapyrroles Macrocycles Phenyl compounds