terbufos on:
[Wikipedia]
[Google]
[Amazon]
Terbufos is a


http://www.inchem.org/pages/ehc.html
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
used in insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to b ...
s and nematicide
A nematicide is a type of chemical pesticide used to kill plant-parasitic nematodes. Nematicides have tended to be broad-spectrum toxicants possessing high volatility or other properties promoting migration through the soil. Aldicarb (Temik), a ca ...
s. Terbufos is part of the chemical family of organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered a ...
s. It is a clear, colourless to pale yellow or reddish-brown liquid and sold commercially as granulate.
History
Terbufos is used on various crops including bananas, beans, citrus, coffee, groundnuts, sorghum, potatoes, sunflowers and maize as soil cover to combat wireworms, mossy beetles, beet flies and the black bean louse. It is not approved for use in theEuropean Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been des ...
. Also the World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
classifies terbufos as a class Ia compound, meaning that terbufos is extremely hazardous. The maximum residue limit
The maximum residue limit (also maximum residue level, MRL), is the maximum amount of pesticide residue that is expected to remain on food products when a pesticide is used according to label directions, that will not be a concern to human health.
...
in the European Union is 0.01 mg/kg terbufos for most crops and animal products. The compound was first registered in 1974 in the United States, together with a United States patent of organophosphates for use in corn fields to deter corn rootworms. Between 1987 and 1996, an average of about 7.5 million pounds (about 3,400 tons) of the compound was used each year. In November 2006, BASF sold its global Terbufos insecticide business to American AMVAC ( American Vanguard Corporation).
Organophosphate poisoning
Organophosphate poisoning is poisoning due to organophosphates (OPs). Organophosphates are used as insecticides, medications, and nerve agents. Symptoms include increased saliva and tear production, diarrhea, vomiting, small pupils, sweating, mus ...
is not common in the developed world, most cases of terbufos poisoning occur in the developing world, where protection against pesticides is scarce, but compounds such as terbufos are widespread, uncontrolled by a government and readily available for farmers.
Available forms
Terbufos is available in granules for application in the agricultural sector. The compound is applied at planting in a band or on the seed furrow directly.Structure and reactivity
Structure
Terbufos, also known as S-((''tert''-butylthio)methyl) O,O-diethyl phosphorodithioate'','' is a compound classified as an organophosphate. Terbufos consists of a centralphosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
atom, surrounded by four different groups. This central atom is surrounded by two ethoxy groups, one double-bonded sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
atom and a (''tert''-butylthio)methanethiol group.
Reactivity
Terbufos is practically insoluble in water, but can be dissolved freely in organic compounds. It decomposes after prolonged heating at 120 °CEintrag zu ''Terbufos'' in der Hazardous Substances Data Bank, retrieved 19 August 2012. and can be hydrolysed when exposed to strong bases (pH>9) and acids (pH<2).Synthesis
The three main compounds that are used for producing terbufos are diethyl-phosphorodithioic acid,formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
and tert-butylthiol
''tert''-Butylthiol, also known as 2-methylpropane-2-thiol, 2-methyl-2-propanethiol, ''tert''-butyl mercaptan (TBM), and ''t''-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which ...
. Formaldehyde acts as a carbon donor between the diethyl-phosphorodithioic acid and tert-butylthiol groups in order to link them. A secondary product of this reaction is water.
Mechanism of action
Terbufos like other organophosphates inactivates theacetylcholinesterase
Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme
Enzymes () are proteins that a ...
in humans by phosphorylation of the hydroxyl group of serine present at the active site of the enzyme.
Metabolism
Metabolism in animals
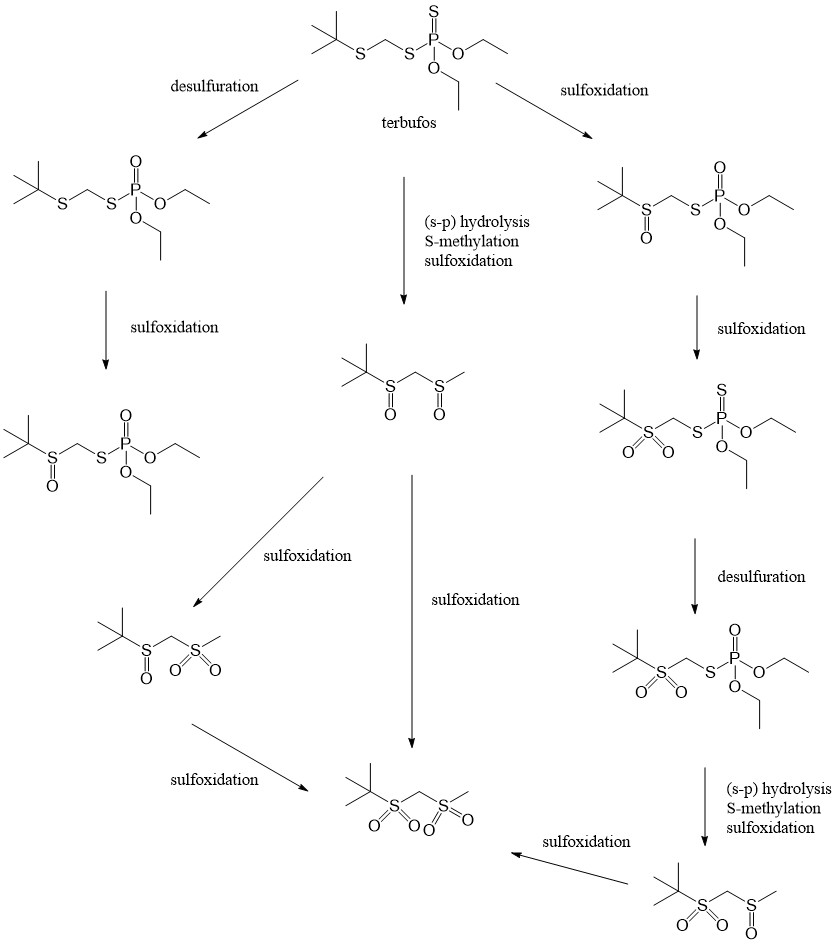
The major excretion route of terbufos in lactating goats, is via the urine. 96.0% and 86.9% of the administered compound was excreted through this route. Neither terbufos, nor a phosphorylated metabolite of the compound was found in milk, and no phosphorylated metabolite was detected in the tissues. A low concentration of terbufos was detected in the liver and the kidneys. Terbufos is extensively metabolised, judging from the low levels of terbufos and its metabolites detected in goat tissues. A proposed metabolism pathway of terbufos suggests a hydrolysis of the thiolophosphorus bond, an enzymatic S-methylation, desulfuration and sulfoxidation occurred in succession. In rats, the proposed metabolism pathway includes more steps, while the metabolic product is the same as proposed in goats. The mechanism in rats includes extra steps in the metabolism of terbufos, and includes more metabolites.
Biotransformation
Terbufos is activated by a biotransformation to asulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
compound. This conversion can take place in the (cellular) environment but also in exposed organisms using the cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
action. This conversion process makes the molecule much more efficient in binding with AChE
Ache or Aches may refer to:
Ethnography
* Aché, an indigenous people of eastern Paraguay
* Aché language, the language of the Aché people
* Ache language (China)
* Aṣẹ (Cuban spelling: ''aché''), a concept in Orisha belief
People
* Ach ...
. The converted form may be significantly more toxic to amphibians than the parent compound.

Toxicity
General Toxicity
Terbufos can enter the body through dermal absorption (skin contact), by inhalation or ingestion of the compound. Studies have suggested that the human NOEL ≥0.009 mg/m^3.Metabolite Toxicity
Two metabolites of terbufos have been tested for toxicity. Terbufos sulfoxide and terbufos sulfone both inhibited cholinesterase activity, but did not cause any mortalities in beagle dogs.Health Effects
Acute effects
Terbufos can induce death by causing an acute cholinergic crisis (ACC). Due to the irreversible inhibition of the AChE enzyme by the compound,acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Part ...
(ACh) can no longer be sufficiently broken down by the AChE. This results in excess ACh, which causes overstimulation of the neuromuscular junction
A neuromuscular junction (or myoneural junction) is a chemical synapse between a motor neuron and a muscle fiber.
It allows the motor neuron to transmit a signal to the muscle fiber, causing muscle contraction.
Muscles require innervation to ...
. The inhibiting effect of terbufos on AChE works on the peripheral muscarinic, nicotinic synapses and the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
. The onset of an ACC can vary from minutes to multiple hours post-exposure.
Symptoms of a terbufos induced ACC result in muscarinic (diaphoresis
Perspiration, also known as sweating, is the production of fluids secreted by the sweat glands in the skin of mammals.
Two types of sweat glands can be found in humans: eccrine glands and apocrine glands. The eccrine sweat glands are distribut ...
, vomiting, miosis, salivation), nicotinic (pallor
Pallor is a pale color of the skin that can be caused by illness, emotional shock or stress, stimulant use, or anemia, and is the result of a reduced amount of oxyhaemoglobin and may also be visible as pallor of the conjunctivae of the eyes o ...
and muscle weakness with respiratory failure) and CNS poisoning (headache, dizziness, altered level of consciousness) symptoms. The toxic effects can be managed by early recognition of terbufos poisoning, rapid decontamination and treatment with atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given i ...
or oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted ...
compounds.
Long-term effects
Long term exposure effects which are specific for terbufos (effects generally associated with Organophosphates (OP's) not included) are: The development of Lung cancer, Leukemia and non-Hodgkin lymphoma (NHL) overall, as well as specific NHL subtypes. In males an increase in aggressive prostate cancer has been also been observed, while in females a non-significant increase in breast cancer can be seen. No genotoxic effects were detected in vitro and in vivo. No developmental abnormalities were noted in research, but a reduced fetal body weight was observed in mammals.Detection
The amount of metabolites, caused by hydrolysis in vivo of an organophosphate compound such as terbufos, can be detected in the urine using gas chromatography and combined gas chromatography/mass spectrometry (GC-MC). Usually it is necessary to preserve the sample of the urine by addition of chloroform, to concentrate or extract the metabolites and to convert them to suitably-volatile derivatives. The detection can be useful to determine patterns of exposure. But the levels of metabolite alone cannot be considered a guide to hazard.WHO; Environ Health Criteria 63: Organophosphorus pesticides (1986). Available from, as of July 22, 2003:References
External links
* {{Acetylcholine metabolism and transport modulators Acetylcholinesterase inhibitors Organophosphate insecticides Phosphorodithioates Nematicides Tert-butyl compounds