synthetic molecular motor on:
[Wikipedia]
[Google]
[Amazon]
 Synthetic molecular motors are
Synthetic molecular motors are
 The motor by Kelly and co-workers is an elegant example of how
The motor by Kelly and co-workers is an elegant example of how 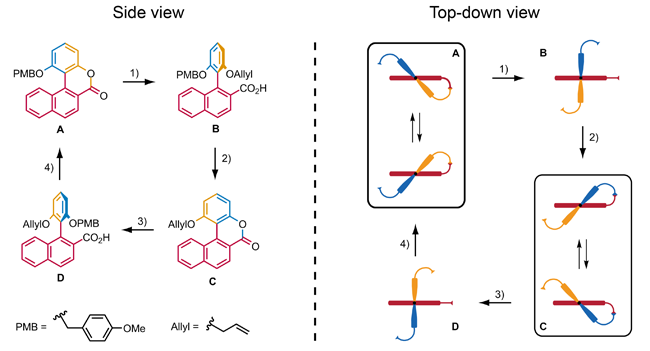 Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation. The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the
Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation. The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the
 Synthetic molecular motors are
Synthetic molecular motors are molecular machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for l ...
s capable of continuous directional rotation under an energy input. Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion (via protein dynamics Proteins are generally thought to adopt unique structures determined by their amino acid sequences. However, proteins are not strictly static objects, but rather populate ensembles of (sometimes similar) conformations. Transitions between these sta ...
), some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors.
The basic requirements for a synthetic motor are repetitive 360° motion, the consumption of energy and unidirectional rotation. The first two efforts in this direction, the chemically driven motor by Dr. T. Ross Kelly of Boston College
Boston College (BC) is a private Jesuit research university in Chestnut Hill, Massachusetts. Founded in 1863, the university has more than 9,300 full-time undergraduates and nearly 5,000 graduate students. Although Boston College is classifie ...
with co-workers and the light-driven motor by Ben Feringa
Bernard Lucas Feringa (, born 18 May 1951) is a Dutch synthetic organic chemist, specializing in molecular nanotechnology and homogeneous catalysis. He is the Jacobus van 't Hoff Distinguished Professor of Molecular Sciences, at the Strating ...
and co-workers, were published in 1999 in the same issue of Nature
Nature, in the broadest sense, is the physical world or universe. "Nature" can refer to the phenomena of the physical world, and also to life in general. The study of nature is a large, if not the only, part of science. Although humans are p ...
.
As of 2020, the smallest atomically precise molecular machine has a rotor that consists of four atoms.
Chemically driven rotary molecular motors
An example of a prototype for a synthetic chemically driven rotary molecular motor was reported by Kelly and co-workers in 1999. Their system is made up from a three-bladedtriptycene
Triptycene is an aromatic hydrocarbon, the simplest iptycene molecule with the formula C2H2(C6H4)3. It is a white solid that is soluble in organic solvents. The compound has a paddle-wheel configuration with D3h symmetry. It is named after the m ...
rotor and a helicene
In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped chiral molecules. The chemistry of helicenes has attracted continuin ...
, and is capable of performing a unidirectional 120° rotation.
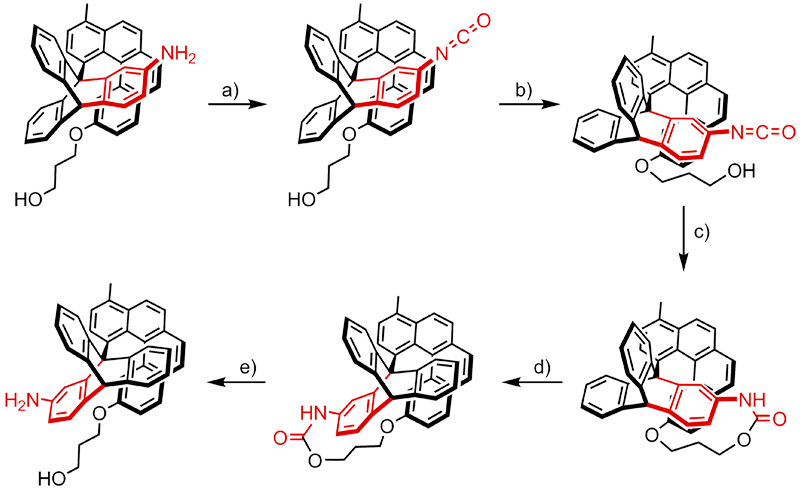
This rotation takes place in five steps. The amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent suc ...
group present on the triptycene moiety is converted to an isocyanate group by condensation with phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, esp ...
(a). Thermal or spontaneous rotation around the central bond then brings the isocyanate group in proximity of the hydroxyl group located on the helicene moiety (b), thereby allowing these two groups to react with each other (c). This reaction irreversibly traps the system as a strained cyclic urethane Urethane may refer to:
*Ethyl carbamate, a chemical compound which is an ester of carbamic acid
*Polyurethane, a polymer composed of a chain of organic units joined by carbamate (urethane) links
*Carbamate
In organic chemistry, a carbamate is a ...
that is higher in energy and thus energetically closer to the rotational energy barrier than the original state. Further rotation of the triptycene moiety therefore requires only a relatively small amount of thermal activation in order to overcome this barrier, thereby releasing the strain (d). Finally, cleavage of the urethane group restores the amine and alcohol functionalities of the molecule (e).
The result of this sequence of events is a unidirectional 120° rotation of the triptycene moiety with respect to the helicene moiety. Additional forward or backward rotation of the triptycene rotor is inhibited by the helicene moiety, which serves a function similar to that of the pawl of a ratchet. The unidirectionality of the system is a result from both the asymmetric skew of the helicene moiety as well as the strain of the cyclic urethane which is formed in c. This strain can be only be lowered by the clockwise rotation of the triptycene rotor in d, as both counterclockwise rotation as well as the inverse process of d are energetically unfavorable. In this respect the preference for the rotation direction is determined by both the positions of the functional groups and the shape of the helicene and is thus built into the design of the molecule instead of dictated by external factors.
 The motor by Kelly and co-workers is an elegant example of how
The motor by Kelly and co-workers is an elegant example of how chemical energy
Chemical energy is the energy of chemical substances that is released when they undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (2018). "How ...
can be used to induce controlled, unidirectional rotational motion, a process which resembles the consumption of ATP in organisms in order to fuel numerous processes. However, it does suffer from a serious drawback: the sequence of events that leads to 120° rotation is not repeatable. Kelly and co-workers have therefore searched for ways to extend the system so that this sequence can be carried out repeatedly. Unfortunately, their attempts to accomplish this objective have not been successful and currently the project has been abandoned. In 2016 David Leigh's group invented the first autonomous chemically-fuelled synthetic molecular motor.
Some other examples of synthetic chemically driven rotary molecular motors that all operate by sequential addition of reagents have been reported, including the use of the stereoselective ring opening of a racemic biaryl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
lactone by the use of chiral reagents, which results in a directed 90° rotation of one aryl with respect to the other aryl. Branchaud and co-workers have reported that this approach, followed by an additional ring closing step, can be used to accomplish a non-repeatable 180° rotation.
 Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation. The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the
Feringa and co-workers used this approach in their design of a molecule that can repeatably perform 360° rotation. The full rotation of this molecular motor takes place in four stages. In stages A and C rotation of the aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
is restricted, although helix
A helix () is a shape like a corkscrew or spiral staircase. It is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as two intertwined helices ...
inversion is possible. In stages B and D the aryl can rotate with respect to the naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
with steric interactions preventing the aryl from passing the naphthalene. The rotary cycle consists of four chemically induced steps which realize the conversion of one stage into the next. Steps 1 and 3 are asymmetric ring opening reactions which make use of a chiral reagent in order to control the direction of the rotation of the aryl. Steps 2 and 4 consist of the deprotection
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
of the phenol, followed by regioselective
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
ring formation.
Light-driven rotary molecular motors
In 1999 the laboratory of Prof. Dr. Ben L. Feringa at the University of Groningen, The Netherlands, reported the creation of a unidirectional molecular rotor. Their 360° molecular motor system consists of a bis-helicene
In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped chiral molecules. The chemistry of helicenes has attracted continuin ...
connected by an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
double bond displaying axial chirality
Axial may refer to:
* one of the anatomical directions describing relationships in an animal body
* In geometry:
:* a geometric term of location
:* an axis of rotation
* In chemistry, referring to an axial bond
* a type of modal frame, in music ...
and having two stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups c ...
s.
One cycle of unidirectional rotation takes 4 reaction steps. The first step is a low temperature endothermic
In thermochemistry, an endothermic process () is any thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015).''Principle of Modern Chemistry'', Brooks Cole. p. ...
photoisomerization of the trans (''P'',''P'') isomer 1 to the ''cis'' (''M'',''M'') 2 where ''P'' stands for the right-handed helix
A helix () is a shape like a corkscrew or spiral staircase. It is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as two intertwined helices ...
and ''M'' for the left-handed helix. In this process, the two axial
Axial may refer to:
* one of the anatomical directions describing relationships in an animal body
* In geometry:
:* a geometric term of location
:* an axis of rotation
* In chemistry, referring to an axial bond
* a type of modal frame, in music
* ...
methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in man ...
groups are converted into two less sterically
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
favorable equatorial methyl groups.
By increasing the temperature to 20 °C these methyl groups convert back exothermally to the (''P'',''P'') ''cis'' axial groups (3) in a helix inversion. Because the axial isomer is more stable than the equatorial isomer, reverse rotation is blocked. A second photoisomerization converts (''P'',''P'') cis 3 into (''M'',''M'') trans 4, again with accompanying formation of sterically unfavorable equatorial methyl groups. A thermal isomerization process at 60 °C closes the 360° cycle back to the axial positions.
A major hurdle to overcome is the long reaction time for complete rotation in these systems, which does not compare to rotation speeds displayed by motor proteins in biological systems. In the fastest system to date, with a fluorene
Fluorene , or 9''H''-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It has a violet fluorescence, hence its name. For commercial p ...
lower half, the half-life of the thermal helix inversion is 0.005 seconds. This compound is synthesized using the Barton-Kellogg reaction. In this molecule the slowest step in its rotation, the thermally induced helix-inversion, is believed to proceed much more quickly because the larger ''tert''-butyl group makes the unstable isomer even less stable than when the methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in man ...
group is used. This is because the unstable isomer is more destabilized than the transition state that leads to helix-inversion. The different behaviour of the two molecules is illustrated by the fact that the half-life time for the compound with a methyl group instead of a ''tert''-butyl group is 3.2 minutes.
The Feringa principle has been incorporated into a prototype nanocar. The car synthesized has a helicene-derived engine with an oligo (phenylene ethynylene) chassis and four carborane wheels and is expected to be able to move on a solid surface with scanning tunneling microscopy monitoring, although so far this has not been observed. The motor does not perform with fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
wheels because they quench the photochemistry of the motor moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
. Feringa motors have also been shown to remain operable when chemically attached to solid surfaces. The ability of certain Feringa systems to act as an asymmetric catalyst
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molec ...
has also been demonstrated.
In 2016, Feringa was awarded a Nobel prize for his work on molecular motors.
Experimental demonstration of a single-molecule electric motor
A single-molecule electrically operated motor made from a single molecule of ''n''-butyl methyl sulfide (C5H12S) has been reported. The molecule is adsorbed onto a copper (111)single-crystal
In materials science, a single crystal (or single-crystal solid or monocrystalline solid) is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries.RIWD. "Re ...
piece by chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like cor ...
.
See also
*Molecular machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for l ...
* Molecular motors
* Molecular propeller
* Nanomotor
A nanomotor is a molecular or nanoscale device capable of converting energy into movement. It can typically generate forces on the order of piconewtons.
While nanoparticles have been utilized by artists for centuries, such as in the famous Lycurg ...
References
{{reflist, 30em Nanotechnology Molecular machines