Supermicelle on:
[Wikipedia]
[Google]
[Amazon]
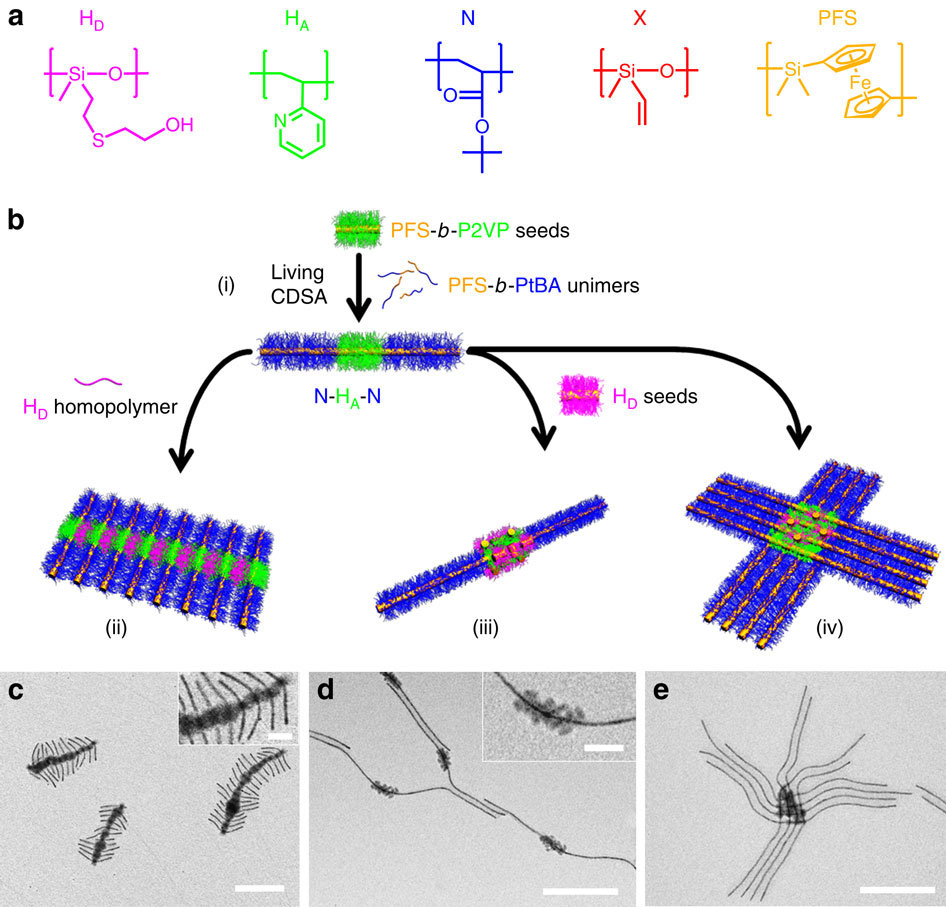 Supermicelle is a hierarchical
Supermicelle is a hierarchical
 Supermicelle is a hierarchical
Supermicelle is a hierarchical micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated collo ...
structure (supramolecular assembly
In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules (e.g., a DNA double helix or an inclusion compound), or a defined num ...
) where individual components are also micelles. Supermicelles are formed via bottom-up chemical approaches, such as self-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the ...
of long cylindrical micelles into radial cross-, star- or dandelion
''Taraxacum'' () is a large genus of flowering plants in the family Asteraceae, which consists of species commonly known as dandelions. The scientific and hobby study of the genus is known as taraxacology. The genus is native to Eurasia and Nor ...
-like patterns in a specially selected solvent; solid nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 1 ...
s may be added to the solution to act as nucleation centers and form the central core of the supermicelle. The stems of the primary cylindrical micelles are composed of various block copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
s connected by strong covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s; within the supermicelle structure they are loosely held together by hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s, electrostatic or solvophobic Solvophobic theory attempts to explain interactions between polar solvents and non-polar solutes. In the pure solvent, there are relatively strong cohesive forces between the solvent molecules due to hydrogen bonding or other polar interactions. He ...
interactions.
References
{{Reflist, refs= {{cite journal, doi=10.1038/ncomms10009, pmid=26627644, title=Transformation and patterning of supermicelles using dynamic holographic assembly, journal=Nature Communications, volume=6, page=10009, year=2015, last1=Gould, first1=Oliver E.C., last2=Qiu, first2=Huibin, last3=Lunn, first3=David J., last4=Rowden, first4=John, last5=Harniman, first5=Robert L., last6=Hudson, first6=Zachary M., last7=Winnik, first7=Mitchell A., last8=Miles, first8=Mervyn J., last9=Manners, first9=Ian, pmc=4686664, bibcode=2015NatCo...610009G {{cite journal, doi=10.1038/ncomms9127, pmid=26337527, pmc=4569713, title=Non-covalent synthesis of supermicelles with complex architectures using spatially confined hydrogen-bonding interactions, journal=Nature Communications, volume=6, page=8127, year=2015, last1=Li, first1=Xiaoyu, last2=Gao, first2=Yang, last3=Boott, first3=Charlotte E., last4=Winnik, first4=Mitchell A., last5=Manners, first5=Ian, bibcode=2015NatCo...6E8127L Supramolecular chemistry Colloidal chemistry