Shapiro Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Shapiro reaction or tosylhydrazone decomposition is an 

 Importantly, the Shapiro reaction cannot be used to synthesize 1-lithioalkenes (and the resulting functionalized derivatives), as sulfonylhydrazones derived from aldehydes undergo exclusive addition of the organolithium base to the carbon of the C–N double bond.
Importantly, the Shapiro reaction cannot be used to synthesize 1-lithioalkenes (and the resulting functionalized derivatives), as sulfonylhydrazones derived from aldehydes undergo exclusive addition of the organolithium base to the carbon of the C–N double bond.


organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
in which a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
or aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
is converted to an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
through an intermediate hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehyde ...
in the presence of 2 equivalents of organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
. The reaction was discovered by Robert H. Shapiro in 1967. The Shapiro reaction was used in the Nicolaou Taxol total synthesis. This reaction is very similar to the Bamford–Stevens reaction The Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens (1900–2000). ...
, which also involves the basic decomposition of tosyl hydrazones.
Reaction mechanism
In a prelude to the actual Shapiro reaction, aketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
or an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
(1) is reacted with ''p''-toluenesulfonylhydrazide(2) to form a ''p''-toluenesulfonylhydrazone (or tosylhydrazone A tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=N-NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.
...
) which is a hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehyde ...
(3). Two equivalents of strong base such as ''n''-butyllithium abstract the proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
from the hydrazone (4) followed by the less acidic
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
proton α to the hydrazone carbon (5), forming a carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
. The carbanion then undergoes an elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
producing a carbon–carbon double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
and ejecting the tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a Toluene, tolyl group, –, joined to a sulfonyl group, ––, with the open vale ...
anion, forming a diazonium
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
General properti ...
anion (6). This diazonium anion is then lost as molecular nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seven ...
resulting in a vinyllithium species (7), which can then be reacted with various electrophiles
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
, including simple neutralization with water or an acid (8).
:
Scope
The position of the alkene in the product is controlled by the site of deprotonation by the organolithium base. In general, the kinetically favored, less substituted site of differentially substituted tosylhydrazones is deprotonated selectively, leading to the less substitutedvinyllithium
Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds..
Preparation and structure
So ...
intermediate. Although many secondary reactions exist for the vinyllithium functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
, in the Shapiro reaction in particular water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
is added, resulting in protonation to the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
. Other reactions of vinyllithium compounds include alkylation reactions with for instance alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely ...
s.
Catalytic Shapiro reaction
Traditional Shapiro reactions require stoichiometric (sometimes excess) amounts of base to generate the alkenyllithium reagents. To combat this problem, Yamamoto and coworkers developed an efficient stereoselective and regioselective route to alkenes using a combination of ketone phenylaziridinylhydrazones as arenesulfonylhydrazone equivalents with a catalytic amount of lithium amides. The required phenylaziridinylhydrazone was prepared from the condensation of undecan-6-one with 1-amino-2-phenylaziridine. Treatment of the phenylaziridinylhydrazone with 0.3 equivalents of LDA in ether resulted in the alkene shown below with a ''cis'':''trans'' ratio of 99.4:0.6. The ratio was determined by capillary GLC analysis after conversion to the corresponding epoxides with mCPBA. The catalyst loading can be reduced to 0.05 equivalents in the case of a 30mmol scale reaction. The high stereoselectivity is obtained by the preferential abstraction of the α-methylene hydrogen syn to the phenylaziridine, and is also accounted for by the internal chelation of the lithiated intermediated.
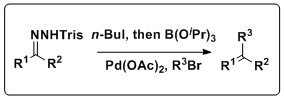
A one pot in situ combined Shapiro-Suzuki reaction
The Shapiro reaction can also be combined with theSuzuki reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, ...
to produce a variety of olefin products. Keay and coworkers have developed methodology that combines these reactions in a one pot process that does not require the isolation of the boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
, a setback of the traditional Suzuki coupling. This reaction has a wide scope, tolerating a slew of trisylhydrazones and aryl halides, as well as several solvents and Pd sources.

An application of the Shapiro reaction in total synthesis
The Shapiro reaction has been used to generate olefins towards to complex natural products. K. Mori and coworkers wanted to determine the absolute configuration of the phytocassane group of a class of natural products calledphytoalexin
Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined, not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized ''de novo'' by plants t ...
s. This was accomplished by preparing the naturally occurring (–)-phytocassane D from (''R'')- Wieland-Miescher ketone. On the way to (–)-phytocassane D, a tricyclic ketone was subjected to Shapiro reaction conditions to yield the cyclic alkene product.

See also
*Hydrazone iodination Hydrazone iodination is an organic reaction in which a hydrazone is converted into a vinyl iodide by reaction of iodine and a non-nucleophilic base such as DBU. First published by Derek Barton in 1962 the reaction is sometimes referred to as the ...
*Wolff–Kishner reduction
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has se ...
References
{{Alkenes Carbon-carbon bond forming reactions Olefination reactions Organic redox reactions Name reactions